The solubility of a gas in H2O at a temperature of 25 °C and a pressure...
The solubility of a gas in H2O at a temperature of 25
°C and a pressure of 0.65 atm is 0.125 M. What will the solubility
(in atm) of the same gas be in H2O at the same
temperature at 3.7 atm?
Homework Answers
The Henry's law constant "k" is different for every gas, temperature and solvent. The units on "k" depend on the units used for concentration and pressure.
The value for k is the same for the same temperature, gas and solvent. This means the concentration to pressure ratio is the same when pressures change. The following equation can be used to relate pressure and concentration changes for solutions at the same temperature. The initial condition has concentration C1 and gas partial pressure P1. The second condition has concentration C2 and gas partial pressure P2.
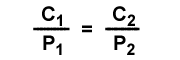 ,
,
0.125/0.65= C2/3.7
C2=0.125x3.7/0.65=0.7115 M
Add Answer to:
The solubility of a gas in H2O at a temperature of 25
°C and a pressure...
need #7-10 answered 6. The solubility of nitrogen gas at 25°C and a nitrogen pressure of...
 need #7-10 answered
6. The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 x 10 mol/L. What is the value of the Henry's Law constant in mol/L-atm? A) 6.8 × 10-4 mol/L-atm B) 4.7 x 10 mol/L-atm C) 3.2 × 104 moVL-atm D) 9.0 x 10 mol/L atm E) 1.5 x 10mol/L atm 7. According to Raoult's law, which statement is false? A) The vapor pressure of a solvent over a solution decreases...
need #7-10 answered
6. The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 x 10 mol/L. What is the value of the Henry's Law constant in mol/L-atm? A) 6.8 × 10-4 mol/L-atm B) 4.7 x 10 mol/L-atm C) 3.2 × 104 moVL-atm D) 9.0 x 10 mol/L atm E) 1.5 x 10mol/L atm 7. According to Raoult's law, which statement is false? A) The vapor pressure of a solvent over a solution decreases...
4. The solubility of nitrogen gas in water at 25°C and a partial pressure of N2...
4. The solubility of nitrogen gas in water at 25°C and a partial pressure of N2 of 0.78 atm is 5.5×10–4 mol/L. A. Calculate kH, Henry’s Law constant for nitrogen gas, at this temperature using the solubility at 0.78 atm. S = kH × P
09 A gas with a volume of 525 mL at a temperature of -25 °C is...
 09 A gas with a volume of 525 mL at a temperature of -25 °C is heated to 175 °C. What is the new volume, in milliliters, of the gas if pressure and number of moles are held constant? 010 A gas has a volume of 2.8 L at a temperature of 27 °C. What temperature (°C) is needed to expand the volume to 15 L? (P and n are constant.) a 1971 011 Combined gas law problem: A balloon...
09 A gas with a volume of 525 mL at a temperature of -25 °C is heated to 175 °C. What is the new volume, in milliliters, of the gas if pressure and number of moles are held constant? 010 A gas has a volume of 2.8 L at a temperature of 27 °C. What temperature (°C) is needed to expand the volume to 15 L? (P and n are constant.) a 1971 011 Combined gas law problem: A balloon...
3. At 20 °C, the solubility of oxygen gas in water is 1.38 × 10–3 M...
3. At 20 °C, the solubility of oxygen gas in water is 1.38 × 10–3 M at a partial pressure of 1.0 atm. What is the solubility of O2 in water when its partial pressure is decreased to 0.21 atm at the same temperature?
4. The partial pressure of nitrogen in air is 0.78 atm at 25 °C on a clear day, and the solubility of N2 in water is 5.3...
4. The partial pressure of nitrogen in air is 0.78 atm at 25 °C on a clear day, and the solubility of N2 in water is 5.3 × 10–4 M. What is the partial pressure of N2 when its solubility in water is 1.1 × 10–3 M at the same temperature?
Question 15 4 pts Determine the solubility of CO2 in soda water at 25°C if the...
 Question 15 4 pts Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 4.4 atm. The Henry's law constant for carbon dioxide in water at this temperature is 3.4 x 10-2 M/atm. O 0.65 M 0.57 M O 0.15 M O 0.18 M
Question 15 4 pts Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 4.4 atm. The Henry's law constant for carbon dioxide in water at this temperature is 3.4 x 10-2 M/atm. O 0.65 M 0.57 M O 0.15 M O 0.18 M
14. Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2...
 14. Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.2 afm. The Henry's law constant for carbon dioxide in water at this temperature is 34 10 A. 0.15 M B. 0.57 M C. 0.65 M D. 0.18 M E. 0.29 M
14. Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.2 afm. The Henry's law constant for carbon dioxide in water at this temperature is 34 10 A. 0.15 M B. 0.57 M C. 0.65 M D. 0.18 M E. 0.29 M
Determine the solubility of N2 in water exposed to air at 25°C if the atmospheric pressure...
Determine the solubility of N2 in water exposed to air at 25°C if the atmospheric pressure is 1.2 atm. Assume that the mole fraction of nitrogen is 0.78 in air and the Henry's law constant for nitrogen in water at this temperature is 6.1 × 10-4 M/atm.
A gas sample had an inknown pressure with a temperature of 42 degrees Celsius. The same...
 A gas sample had an inknown pressure with a temperature of 42
degrees Celsius. The same gas has a pressure of 2.83 atm when the
temperature is -18 degrees Celsius, with no change in volume or
amount of gas. What is the initial pressure, in atm, of the
gas?
Agas sample has a unknown pressure with a temperature of 42°C. The same gas has a pressure of 2.83 atm when the temperature is -18°C with no change in the volume...
A gas sample had an inknown pressure with a temperature of 42
degrees Celsius. The same gas has a pressure of 2.83 atm when the
temperature is -18 degrees Celsius, with no change in volume or
amount of gas. What is the initial pressure, in atm, of the
gas?
Agas sample has a unknown pressure with a temperature of 42°C. The same gas has a pressure of 2.83 atm when the temperature is -18°C with no change in the volume...
The solubility constant for CO2 in water at 25∘C is 3.40 x 10-2 M/atm. The solubility...
 The solubility constant for CO2 in water at
25∘C
is 3.40 x 10-2 M/atm. The solubility of CO2
is 34.164 mM in water that is exposed to a gas mixture in which the
mole fraction of CO2 is 0.78. What is the total
pressure of this gas mixture? Express your answer in units
of atmospheres using at least three significant figures.
The solubility constant for CO2 in water at
25∘C
is 3.40 x 10-2 M/atm. The solubility of CO2
is 34.164 mM in water that is exposed to a gas mixture in which the
mole fraction of CO2 is 0.78. What is the total
pressure of this gas mixture? Express your answer in units
of atmospheres using at least three significant figures.
Most questions answered within 3 hours.
-
Assume that the situation can be expressed as a linear cost
function. Find the cost function....
asked 57 minutes ago -
(Sort ArrayList) Write the following method that sorts an
ArrayList: public static > void sort(ArrayList list)...
asked 1 hour ago -
2) A 9 volt battery is connected to a parallel plate capacitor
with an initial capacitance...
asked 57 minutes ago -
Prince Equipment has to decide whether to obtain $1,000,000 of
financing by: (1) selling common stock...
asked 2 hours ago -
The terms electrical and electronic have similar but
different meanings which of the following statement is...
asked 2 hours ago -
You wish to test the claim that the mean GPA of night students
is than 2.5...
asked 2 hours ago -
A soft drink machine outputs a mean of 25 ounces per cup. The
machine's output is...
asked 3 hours ago -
Ross Co., Westerfield, Inc., and Jordan Company announced a new
agreement to market their respective products...
asked 3 hours ago -
What is the distance run by a runner 50m/hr and
60m/hr?
asked 4 hours ago -
Write a descendant selector rule that affects the <em>
element that is contained within the
<p>...
asked 4 hours ago -
The management of an electrical manufacturing plant determined
that the mean life of their 10 watt...
asked 4 hours ago -
On January 1, Year 1, Prairie Enterprises purchased a parcel of
land for $13,100 cash. At...
asked 5 hours ago
 need #7-10 answered
6. The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 x 10 mol/L. What is the value of the Henry's Law constant in mol/L-atm? A) 6.8 × 10-4 mol/L-atm B) 4.7 x 10 mol/L-atm C) 3.2 × 104 moVL-atm D) 9.0 x 10 mol/L atm E) 1.5 x 10mol/L atm 7. According to Raoult's law, which statement is false? A) The vapor pressure of a solvent over a solution decreases...
need #7-10 answered
6. The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 x 10 mol/L. What is the value of the Henry's Law constant in mol/L-atm? A) 6.8 × 10-4 mol/L-atm B) 4.7 x 10 mol/L-atm C) 3.2 × 104 moVL-atm D) 9.0 x 10 mol/L atm E) 1.5 x 10mol/L atm 7. According to Raoult's law, which statement is false? A) The vapor pressure of a solvent over a solution decreases...
 09 A gas with a volume of 525 mL at a temperature of -25 °C is heated to 175 °C. What is the new volume, in milliliters, of the gas if pressure and number of moles are held constant? 010 A gas has a volume of 2.8 L at a temperature of 27 °C. What temperature (°C) is needed to expand the volume to 15 L? (P and n are constant.) a 1971 011 Combined gas law problem: A balloon...
09 A gas with a volume of 525 mL at a temperature of -25 °C is heated to 175 °C. What is the new volume, in milliliters, of the gas if pressure and number of moles are held constant? 010 A gas has a volume of 2.8 L at a temperature of 27 °C. What temperature (°C) is needed to expand the volume to 15 L? (P and n are constant.) a 1971 011 Combined gas law problem: A balloon...
 Question 15 4 pts Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 4.4 atm. The Henry's law constant for carbon dioxide in water at this temperature is 3.4 x 10-2 M/atm. O 0.65 M 0.57 M O 0.15 M O 0.18 M
Question 15 4 pts Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 4.4 atm. The Henry's law constant for carbon dioxide in water at this temperature is 3.4 x 10-2 M/atm. O 0.65 M 0.57 M O 0.15 M O 0.18 M
 14. Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.2 afm. The Henry's law constant for carbon dioxide in water at this temperature is 34 10 A. 0.15 M B. 0.57 M C. 0.65 M D. 0.18 M E. 0.29 M
14. Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.2 afm. The Henry's law constant for carbon dioxide in water at this temperature is 34 10 A. 0.15 M B. 0.57 M C. 0.65 M D. 0.18 M E. 0.29 M
 A gas sample had an inknown pressure with a temperature of 42
degrees Celsius. The same gas has a pressure of 2.83 atm when the
temperature is -18 degrees Celsius, with no change in volume or
amount of gas. What is the initial pressure, in atm, of the
gas?
Agas sample has a unknown pressure with a temperature of 42°C. The same gas has a pressure of 2.83 atm when the temperature is -18°C with no change in the volume...
A gas sample had an inknown pressure with a temperature of 42
degrees Celsius. The same gas has a pressure of 2.83 atm when the
temperature is -18 degrees Celsius, with no change in volume or
amount of gas. What is the initial pressure, in atm, of the
gas?
Agas sample has a unknown pressure with a temperature of 42°C. The same gas has a pressure of 2.83 atm when the temperature is -18°C with no change in the volume...
 The solubility constant for CO2 in water at
25∘C
is 3.40 x 10-2 M/atm. The solubility of CO2
is 34.164 mM in water that is exposed to a gas mixture in which the
mole fraction of CO2 is 0.78. What is the total
pressure of this gas mixture? Express your answer in units
of atmospheres using at least three significant figures.
The solubility constant for CO2 in water at
25∘C
is 3.40 x 10-2 M/atm. The solubility of CO2
is 34.164 mM in water that is exposed to a gas mixture in which the
mole fraction of CO2 is 0.78. What is the total
pressure of this gas mixture? Express your answer in units
of atmospheres using at least three significant figures.