Homework Answers
6) concentration of methyl amine = 0.35M and kb = 4.4x10-4
Thus pKb of methylamine = -log Kb = 3.356
The pOH of a weak base = 1/2 [pkb - logC]
= 1/2 [3.356 - log 0.35]
= 1.45
Thus pH of the solution = 14 - pOH
= 14-1.45
= 12.55
7) The solubility equilibrium is
Mn(OH)2(s) <-------------> Mn+2(aq) + 2OH- (aq)
- s 2s
Ksp = [Mn+2][OH-]2
= s x (2s)2
= 4s3
Given solubility s = 2.2x10-5 M Thus
Ksp = 4 (2.2x10-5 M )3
= 4.259x10-14
8) The salt whose solubility is most sensitive to pH is CaF2
As CaF2 is a salt of weak acid HF and strong base Ca(OH)2 , itundergoes hydrolysis in aqueous solution and is affected by acid .
The others are salts of strong acids HNO3,HCl and HBr. So their solubility is not affected much by acid.
Add Answer to:
Please show work
6. Determine the pH of a 0.35 M aqueous solution of (methylamine). The...
Determine the pH of a 0.35 M aqueous solution of CH3NH2 (methylamine). The Kb of methylamine...
Determine the pH of a 0.35 M aqueous solution of CH3NH2 (methylamine). The Kb of methylamine is 4.4 × 10−4. can you please show your work and explain the steps on how you get 12.09 as the answer
what is the pH of 0.36M methylamine solution? pH of 0.36 M methylamine sol. (Kb =...
what is the pH of 0.36M methylamine solution? pH of 0.36 M methylamine sol. (Kb = 4.4 * 10^-4)
please show work i need to know this for a test Question 20 1 pts The...
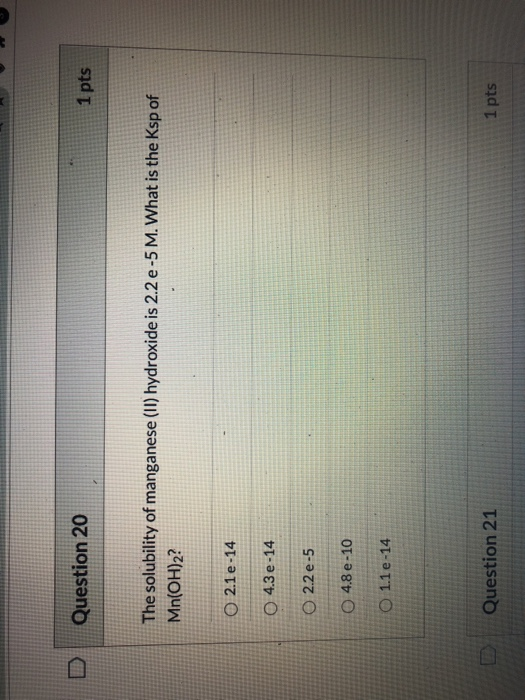 please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
Calculate the pH of a 1.40 M CH3NH3Cl solution. Kb for methylamine, CH3NH2, is 4.4 ×...
Calculate the pH of a 1.40 M CH3NH3Cl solution. Kb for methylamine, CH3NH2, is 4.4 × 10-4. Calculate the pH of a 1.40 M CH3NH3Cl solution. Kb for methylamine, CH3NH2, is 4.4 × 10-4. 1.61 8.75 5.25 12.39
The Effect of a Common Ion on Molar Solubility: pH and OH- as a Common Ion...
 The Effect of a Common Ion on Molar Solubility: pH and
OH- as a Common Ion
We have seen from our study of acid/base chemistry that the
presence of a weak base such as ammonia (Kb = 1.8 X
10-5) can affect the pH of an aqueous solution. This pH
can in turn affect the solubility of a metal hydroxide salt as a
result of the common ion effect. The Ksp for manganese (II)
hydroxide = 2.0 X 10-13. What...
The Effect of a Common Ion on Molar Solubility: pH and
OH- as a Common Ion
We have seen from our study of acid/base chemistry that the
presence of a weak base such as ammonia (Kb = 1.8 X
10-5) can affect the pH of an aqueous solution. This pH
can in turn affect the solubility of a metal hydroxide salt as a
result of the common ion effect. The Ksp for manganese (II)
hydroxide = 2.0 X 10-13. What...
A) Calculate the pH of a 0.0116 M aqueous solution of methylamine (CH3NH2, Kb = 4.2×10-4)...
A) Calculate the pH of a 0.0116 M aqueous solution of methylamine (CH3NH2, Kb = 4.2×10-4) and the equilibrium concentrations of the weak base and its conjugate acid. pH = ? [CH3NH2]equilibrium = ? M [CH3NH3+ ]equilibrium = ? M B)Calculate the pH of a 0.0115 M aqueous solution of nitrous acid (HNO2, Ka= 4.6×10-4) and the equilibrium concentrations of the weak acid and its conjugate base. pH = ? [HNO2]equilibrium = ? M [NO2- ]equilibrium = ? M C)...
Calculate the pH of a solution consisting of 0.225 M solution of CH3NH2 (methylamine) and 0.200...
 Calculate the pH of a solution consisting of 0.225 M solution of CH3NH2 (methylamine) and 0.200 M CH3NH2 tor (methylammonium chloride). Report answer to 2 decimal places. Kb CH3NH2) - 44 x 10-4 CH3NH2 (aq) + H200 + CH3NH3 (2) + OH(aq) Assuming equal initial concentrations of the given species, which of the following is the weakest acid in aqueous solution? O. HB Ky = 2.0 x 10-6 OB.HD K = 6.0 x 10-2 ОС: НЕ Kg = 4.0 x...
Calculate the pH of a solution consisting of 0.225 M solution of CH3NH2 (methylamine) and 0.200 M CH3NH2 tor (methylammonium chloride). Report answer to 2 decimal places. Kb CH3NH2) - 44 x 10-4 CH3NH2 (aq) + H200 + CH3NH3 (2) + OH(aq) Assuming equal initial concentrations of the given species, which of the following is the weakest acid in aqueous solution? O. HB Ky = 2.0 x 10-6 OB.HD K = 6.0 x 10-2 ОС: НЕ Kg = 4.0 x...
27. What is the pH of an aqueous solution that contains 0.35 M of lactic acid,...
 27. What is the pH of an aqueous solution that contains 0.35 M of lactic acid, HC3H5O2, (Ka = 4.4 X 10 -4 ) and 0.55 M of Nat C3H5O2 - ? a. 2.77 b. 3.55 C. 4.65 d. 7.35 e. 5.89
27. What is the pH of an aqueous solution that contains 0.35 M of lactic acid, HC3H5O2, (Ka = 4.4 X 10 -4 ) and 0.55 M of Nat C3H5O2 - ? a. 2.77 b. 3.55 C. 4.65 d. 7.35 e. 5.89
Calculate the (OH) and the pH of a 0.023-M methylamine solution; Kb = 5.0 x 10"....
 Calculate the (OH) and the pH of a 0.023-M methylamine solution; Kb = 5.0 x 10". [OH-] =D pH =
Calculate the (OH) and the pH of a 0.023-M methylamine solution; Kb = 5.0 x 10". [OH-] =D pH =
please explain your answer 3. An aqueous solution of 0.204 M methylamine, CH3NH2, is hydrochloric acid,...
 please explain your answer
3. An aqueous solution of 0.204 M methylamine, CH3NH2, is hydrochloric acid, HCL. The base ionization constant for methylamine is 4.4 x 10-1. What is the pH of the mixture when 12.50 ml of the methylamine solution has been delivered by the buret? added by buret to 25.00 mL of 0.102 M 50 grade paints] SHOW YOUR WORK AND/OR REASONING
please explain your answer
3. An aqueous solution of 0.204 M methylamine, CH3NH2, is hydrochloric acid, HCL. The base ionization constant for methylamine is 4.4 x 10-1. What is the pH of the mixture when 12.50 ml of the methylamine solution has been delivered by the buret? added by buret to 25.00 mL of 0.102 M 50 grade paints] SHOW YOUR WORK AND/OR REASONING
Most questions answered within 3 hours.
-
Mr. and Mrs. Brown report taxable income of $130,000 in 2018. In
addition, they report the...
asked 38 minutes ago -
You capture data by having 8 people fill in 30 small dots on a
piece of...
asked 4 hours ago -
What are the z-values such that 70% of the area lies in the
middle of the...
asked 6 hours ago -
You borrow $29,500 to purchase a brand new car. The interest
rate is 6%, and the...
asked 5 hours ago -
The amount of time it takes an asteroid, whose average distance
from the Sun is 4.70...
asked 6 hours ago -
A 0.289 kg mass slides on a frictionless floor with a speed of
1.34 m/s. The...
asked 6 hours ago -
15.A box contains five red balls, three green balls, and two
yellow balls. Suppose you select...
asked 9 hours ago -
Assume you are given the following for Stackelberg industries:
Return on Assets (ROA) = 8%
Debt...
asked 7 hours ago -
an engineer proposes to use mmWave in 5G network to
increase data rate.
What are the...
asked 7 hours ago -
Explain the idea behind using SEMIJOIN in distributed
query processing.
asked 7 hours ago -
why
is context important in selecting and applying guidelines and
principles for interface design?
illustrate your...
asked 7 hours ago -
In a certain board game, a player rolls two fair six-sided dice
until the player rolls...
asked 8 hours ago

 The Effect of a Common Ion on Molar Solubility: pH and
OH- as a Common Ion
We have seen from our study of acid/base chemistry that the
presence of a weak base such as ammonia (Kb = 1.8 X
10-5) can affect the pH of an aqueous solution. This pH
can in turn affect the solubility of a metal hydroxide salt as a
result of the common ion effect. The Ksp for manganese (II)
hydroxide = 2.0 X 10-13. What...
The Effect of a Common Ion on Molar Solubility: pH and
OH- as a Common Ion
We have seen from our study of acid/base chemistry that the
presence of a weak base such as ammonia (Kb = 1.8 X
10-5) can affect the pH of an aqueous solution. This pH
can in turn affect the solubility of a metal hydroxide salt as a
result of the common ion effect. The Ksp for manganese (II)
hydroxide = 2.0 X 10-13. What...
 Calculate the pH of a solution consisting of 0.225 M solution of CH3NH2 (methylamine) and 0.200 M CH3NH2 tor (methylammonium chloride). Report answer to 2 decimal places. Kb CH3NH2) - 44 x 10-4 CH3NH2 (aq) + H200 + CH3NH3 (2) + OH(aq) Assuming equal initial concentrations of the given species, which of the following is the weakest acid in aqueous solution? O. HB Ky = 2.0 x 10-6 OB.HD K = 6.0 x 10-2 ОС: НЕ Kg = 4.0 x...
Calculate the pH of a solution consisting of 0.225 M solution of CH3NH2 (methylamine) and 0.200 M CH3NH2 tor (methylammonium chloride). Report answer to 2 decimal places. Kb CH3NH2) - 44 x 10-4 CH3NH2 (aq) + H200 + CH3NH3 (2) + OH(aq) Assuming equal initial concentrations of the given species, which of the following is the weakest acid in aqueous solution? O. HB Ky = 2.0 x 10-6 OB.HD K = 6.0 x 10-2 ОС: НЕ Kg = 4.0 x...
 27. What is the pH of an aqueous solution that contains 0.35 M of lactic acid, HC3H5O2, (Ka = 4.4 X 10 -4 ) and 0.55 M of Nat C3H5O2 - ? a. 2.77 b. 3.55 C. 4.65 d. 7.35 e. 5.89
27. What is the pH of an aqueous solution that contains 0.35 M of lactic acid, HC3H5O2, (Ka = 4.4 X 10 -4 ) and 0.55 M of Nat C3H5O2 - ? a. 2.77 b. 3.55 C. 4.65 d. 7.35 e. 5.89
 Calculate the (OH) and the pH of a 0.023-M methylamine solution; Kb = 5.0 x 10". [OH-] =D pH =
Calculate the (OH) and the pH of a 0.023-M methylamine solution; Kb = 5.0 x 10". [OH-] =D pH =
 please explain your answer
3. An aqueous solution of 0.204 M methylamine, CH3NH2, is hydrochloric acid, HCL. The base ionization constant for methylamine is 4.4 x 10-1. What is the pH of the mixture when 12.50 ml of the methylamine solution has been delivered by the buret? added by buret to 25.00 mL of 0.102 M 50 grade paints] SHOW YOUR WORK AND/OR REASONING
please explain your answer
3. An aqueous solution of 0.204 M methylamine, CH3NH2, is hydrochloric acid, HCL. The base ionization constant for methylamine is 4.4 x 10-1. What is the pH of the mixture when 12.50 ml of the methylamine solution has been delivered by the buret? added by buret to 25.00 mL of 0.102 M 50 grade paints] SHOW YOUR WORK AND/OR REASONING