Homework Answers

 please do like if you
understand
please do like if you
understand

 please do like if you
understand
please do like if you
understand

 please do like if you
understand
please do like if you
understand

 please do like if you
understand
please do like if you
understand
Add Answer to:
i need help with 7 & 8?
5. What is meant by "Fractionating column efficiency"? Under...
Consider the phase diagram below for a mixture of chloroform and methanol, looking at how the...
 Consider the phase diagram below for a mixture of chloroform and methanol, looking at how the boiling point changes with composition at constant pressure. If you started with a solution that was Xchloroform = 0.1 at a temperature of 333 K, is it possible to use fractional distillation to purify this solution to nearly 100% chloroform? Approximately what concentration (in mole fraction) would you expect you could achieve through fractional distillation? Curves calculated by mod. UNIFAC (Dortmund) Temperature Minimum Azeotrope...
Consider the phase diagram below for a mixture of chloroform and methanol, looking at how the boiling point changes with composition at constant pressure. If you started with a solution that was Xchloroform = 0.1 at a temperature of 333 K, is it possible to use fractional distillation to purify this solution to nearly 100% chloroform? Approximately what concentration (in mole fraction) would you expect you could achieve through fractional distillation? Curves calculated by mod. UNIFAC (Dortmund) Temperature Minimum Azeotrope...
1. As the mole fraction of chloroform approaches 1, the vapor pressure of acetone could be...
 1. As the mole fraction of chloroform approaches 1, the vapor
pressure of acetone could be calculated using
A. Raoult's Law.
B. Henry's Law.
2.If a chloroform-acetone mixture with an chloroform mole
fraction of 0.62 is subjected to fractional distillation, what is
the composition of the distillate?
A. pure chloroform
B. pure azeotrope
C. pure acetone
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a...
1. As the mole fraction of chloroform approaches 1, the vapor
pressure of acetone could be calculated using
A. Raoult's Law.
B. Henry's Law.
2.If a chloroform-acetone mixture with an chloroform mole
fraction of 0.62 is subjected to fractional distillation, what is
the composition of the distillate?
A. pure chloroform
B. pure azeotrope
C. pure acetone
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a...
1. Explain how a fractionating column increases the separation of two liquids. 2. What is an...
1. Explain how a fractionating column increases the separation of two liquids. 2. What is an azeotrope? Do you think the mixture used in the lab is an example of an azeotrope? Explain. 3. Explain how the boiling point of a liquid is affected by the addition of a non-volatile solute (hint: it is a colligative property). Is the temperature above such solution lower, higher or the same as the pure liquid? Explain why. 4. You were asked to record...
3. Do chloroform and acetone form an ideal solution? A. no B. cannot be determined from...
 3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a chloroform-acetone mixture with an acetone mole fraction
of 0.6 is subjected to fractional distillation, what is the
composition of the distillate?
A. pure azeotrope
B. pure acetone
C. none of the choices shown
D. pure chloroform
5. The acetone-chloroform intermolecular attractions are
_________________ the acetone-acetone or chloroform-chloroform
attractions.
A. less than
B. equal to
C. greater...
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a chloroform-acetone mixture with an acetone mole fraction
of 0.6 is subjected to fractional distillation, what is the
composition of the distillate?
A. pure azeotrope
B. pure acetone
C. none of the choices shown
D. pure chloroform
5. The acetone-chloroform intermolecular attractions are
_________________ the acetone-acetone or chloroform-chloroform
attractions.
A. less than
B. equal to
C. greater...
Please help by doing a step by step solution neatly with explanation :) 8 The feed to a fractionating column operati...
 Please help by doing a step by step solution neatly
with explanation :)
8 The feed to a fractionating column operating at atmospheric pressure has the composition of 60 mole % methanol and 40 mole % water. The top product should be 90 mole % methanol and the bottom product should not contain more than 10 mole %. The vapour. liquid equilibrium data for the methanol-water system at atmospheric pressure is supplied in the table below Equilibrium data for nethanol-water...
Please help by doing a step by step solution neatly
with explanation :)
8 The feed to a fractionating column operating at atmospheric pressure has the composition of 60 mole % methanol and 40 mole % water. The top product should be 90 mole % methanol and the bottom product should not contain more than 10 mole %. The vapour. liquid equilibrium data for the methanol-water system at atmospheric pressure is supplied in the table below Equilibrium data for nethanol-water...
I would appreciated being walked through this, not quite sure how to start this. Part A...
 I would appreciated being walked through this, not
quite sure how to start this.
Part A Predict the boiling point of a mixture made up of 0.70 mole fraction of A and 0.30 mole fraction of B Express your answer as an integer and include the appropriate units. The boiling point of a mixture of two volatile liquids depends on how much of each component is present. This graph shows the boiling temperature versus mole fraction composition. (Figure 1) Fractional...
I would appreciated being walked through this, not
quite sure how to start this.
Part A Predict the boiling point of a mixture made up of 0.70 mole fraction of A and 0.30 mole fraction of B Express your answer as an integer and include the appropriate units. The boiling point of a mixture of two volatile liquids depends on how much of each component is present. This graph shows the boiling temperature versus mole fraction composition. (Figure 1) Fractional...
Course Home <MC4 Fractional Distillation ③ 8 or 12 Review Constants Periodic Table - Part The...
 Course Home <MC4 Fractional Distillation ③ 8 or 12 Review Constants Periodic Table - Part The boiling point of a mocture of two volatile liquids depends on how much of each component is present This graph shows the boiling temperature versus mole fraction composition (Figure 1) Fractional disolation is a physical process used to separate the volatile components of a mixture. The process is accomplished Predict the mole fraction of B in the vapor produced when a mixture of 0.70...
Course Home <MC4 Fractional Distillation ③ 8 or 12 Review Constants Periodic Table - Part The boiling point of a mocture of two volatile liquids depends on how much of each component is present This graph shows the boiling temperature versus mole fraction composition (Figure 1) Fractional disolation is a physical process used to separate the volatile components of a mixture. The process is accomplished Predict the mole fraction of B in the vapor produced when a mixture of 0.70...
I need help with problem 16. Not problem 16 under chapter 13 set 5. *9. 9....
 I need help with problem 16. Not problem 16 under chapter 13 set
5.
*9. 9. The vapor pressure of pure toluene, CyHx, is 137 torr at 60°C. What is the vapor pressure of toluene in a solution made by dissolving 25.0 grams of the nonelectrolyte glucose, C6H12O6, in 50.0 grams of toluene at 60°C? 10. The vapor pressure of water is 118 torr at 55°C. How many grams of urea, CO(NH2)2 must be added to 67.4 grams of water...
I need help with problem 16. Not problem 16 under chapter 13 set
5.
*9. 9. The vapor pressure of pure toluene, CyHx, is 137 torr at 60°C. What is the vapor pressure of toluene in a solution made by dissolving 25.0 grams of the nonelectrolyte glucose, C6H12O6, in 50.0 grams of toluene at 60°C? 10. The vapor pressure of water is 118 torr at 55°C. How many grams of urea, CO(NH2)2 must be added to 67.4 grams of water...
Most questions answered within 3 hours.
-
1.How large must the coefficient of static friction be between
the tires and the road if...
asked 10 minutes ago -
What is the time complexity (Big-O) of the following code?
class Main
{
// Recursive...
asked 10 minutes ago -
Economists look at any situation in terms of its component
parts: the people making decisions, the...
asked 16 minutes ago -
What is a population?
Select one:
a. All of the individual organisms belonging to the same...
asked 20 minutes ago -
You have a yeast cell culture with a concentration of 5x10^4
cells/ml. If you dilute this...
asked 24 minutes ago -
In which direction the Reaction goes? Show detailed process.
SeO3 + 2ClO2. + 2H3O <---> Se...
asked 37 minutes ago -
Unexposed silver halides are removed from photographic film when
they react with sodium thiosulfate
(Na2S2O3, called...
asked 38 minutes ago -
A 0.3054 gram sample of the mineral chalcopyrite (CuFeS2)
yielded 0.6525 gram BaSO4 precipitate. What is...
asked 38 minutes ago -
An short-seller in Tesla is worried the latest management
earnings forecast is too aggressive and the...
asked 1 hour ago -
Question 3 (1 point)
Fill in the blank. Speed Car Rental company found that the tire...
asked 1 hour ago -
1. A copper wire is 26.61 cm long and weighs 1.265 g. The
density of copper...
asked 1 hour ago -
Remember that a concept sketch consists of a sketch (or
series of sketches), labels, and complete...
asked 1 hour ago

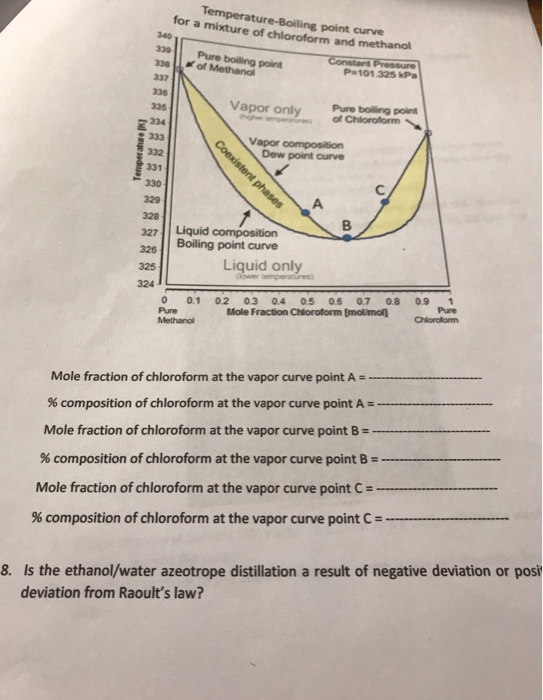
 Consider the phase diagram below for a mixture of chloroform and methanol, looking at how the boiling point changes with composition at constant pressure. If you started with a solution that was Xchloroform = 0.1 at a temperature of 333 K, is it possible to use fractional distillation to purify this solution to nearly 100% chloroform? Approximately what concentration (in mole fraction) would you expect you could achieve through fractional distillation? Curves calculated by mod. UNIFAC (Dortmund) Temperature Minimum Azeotrope...
Consider the phase diagram below for a mixture of chloroform and methanol, looking at how the boiling point changes with composition at constant pressure. If you started with a solution that was Xchloroform = 0.1 at a temperature of 333 K, is it possible to use fractional distillation to purify this solution to nearly 100% chloroform? Approximately what concentration (in mole fraction) would you expect you could achieve through fractional distillation? Curves calculated by mod. UNIFAC (Dortmund) Temperature Minimum Azeotrope...
 1. As the mole fraction of chloroform approaches 1, the vapor
pressure of acetone could be calculated using
A. Raoult's Law.
B. Henry's Law.
2.If a chloroform-acetone mixture with an chloroform mole
fraction of 0.62 is subjected to fractional distillation, what is
the composition of the distillate?
A. pure chloroform
B. pure azeotrope
C. pure acetone
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a...
1. As the mole fraction of chloroform approaches 1, the vapor
pressure of acetone could be calculated using
A. Raoult's Law.
B. Henry's Law.
2.If a chloroform-acetone mixture with an chloroform mole
fraction of 0.62 is subjected to fractional distillation, what is
the composition of the distillate?
A. pure chloroform
B. pure azeotrope
C. pure acetone
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a...
 3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a chloroform-acetone mixture with an acetone mole fraction
of 0.6 is subjected to fractional distillation, what is the
composition of the distillate?
A. pure azeotrope
B. pure acetone
C. none of the choices shown
D. pure chloroform
5. The acetone-chloroform intermolecular attractions are
_________________ the acetone-acetone or chloroform-chloroform
attractions.
A. less than
B. equal to
C. greater...
3. Do chloroform and acetone form an ideal solution?
A. no
B. cannot be determined from the information given
C. yes
4. If a chloroform-acetone mixture with an acetone mole fraction
of 0.6 is subjected to fractional distillation, what is the
composition of the distillate?
A. pure azeotrope
B. pure acetone
C. none of the choices shown
D. pure chloroform
5. The acetone-chloroform intermolecular attractions are
_________________ the acetone-acetone or chloroform-chloroform
attractions.
A. less than
B. equal to
C. greater...
 Please help by doing a step by step solution neatly
with explanation :)
8 The feed to a fractionating column operating at atmospheric pressure has the composition of 60 mole % methanol and 40 mole % water. The top product should be 90 mole % methanol and the bottom product should not contain more than 10 mole %. The vapour. liquid equilibrium data for the methanol-water system at atmospheric pressure is supplied in the table below Equilibrium data for nethanol-water...
Please help by doing a step by step solution neatly
with explanation :)
8 The feed to a fractionating column operating at atmospheric pressure has the composition of 60 mole % methanol and 40 mole % water. The top product should be 90 mole % methanol and the bottom product should not contain more than 10 mole %. The vapour. liquid equilibrium data for the methanol-water system at atmospheric pressure is supplied in the table below Equilibrium data for nethanol-water...
 I would appreciated being walked through this, not
quite sure how to start this.
Part A Predict the boiling point of a mixture made up of 0.70 mole fraction of A and 0.30 mole fraction of B Express your answer as an integer and include the appropriate units. The boiling point of a mixture of two volatile liquids depends on how much of each component is present. This graph shows the boiling temperature versus mole fraction composition. (Figure 1) Fractional...
I would appreciated being walked through this, not
quite sure how to start this.
Part A Predict the boiling point of a mixture made up of 0.70 mole fraction of A and 0.30 mole fraction of B Express your answer as an integer and include the appropriate units. The boiling point of a mixture of two volatile liquids depends on how much of each component is present. This graph shows the boiling temperature versus mole fraction composition. (Figure 1) Fractional...
 Course Home <MC4 Fractional Distillation ③ 8 or 12 Review Constants Periodic Table - Part The boiling point of a mocture of two volatile liquids depends on how much of each component is present This graph shows the boiling temperature versus mole fraction composition (Figure 1) Fractional disolation is a physical process used to separate the volatile components of a mixture. The process is accomplished Predict the mole fraction of B in the vapor produced when a mixture of 0.70...
Course Home <MC4 Fractional Distillation ③ 8 or 12 Review Constants Periodic Table - Part The boiling point of a mocture of two volatile liquids depends on how much of each component is present This graph shows the boiling temperature versus mole fraction composition (Figure 1) Fractional disolation is a physical process used to separate the volatile components of a mixture. The process is accomplished Predict the mole fraction of B in the vapor produced when a mixture of 0.70...
 I need help with problem 16. Not problem 16 under chapter 13 set
5.
*9. 9. The vapor pressure of pure toluene, CyHx, is 137 torr at 60°C. What is the vapor pressure of toluene in a solution made by dissolving 25.0 grams of the nonelectrolyte glucose, C6H12O6, in 50.0 grams of toluene at 60°C? 10. The vapor pressure of water is 118 torr at 55°C. How many grams of urea, CO(NH2)2 must be added to 67.4 grams of water...
I need help with problem 16. Not problem 16 under chapter 13 set
5.
*9. 9. The vapor pressure of pure toluene, CyHx, is 137 torr at 60°C. What is the vapor pressure of toluene in a solution made by dissolving 25.0 grams of the nonelectrolyte glucose, C6H12O6, in 50.0 grams of toluene at 60°C? 10. The vapor pressure of water is 118 torr at 55°C. How many grams of urea, CO(NH2)2 must be added to 67.4 grams of water...