Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the...
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.
Homework Answers
Zaitsev rule. It may be possible in some
instances to create a double bond through an alcohol dehydration
reaction in which hydrogen atoms are lost from two different
carbons on the carbocation. The major product is always the more
highly substituted alkene, that is, the alkene with the greater
number of substituents on the carbon atoms of the double bond, an
observation called the Zaitsev rule. Thus, in the dehydration
reaction of 2-butanol, the following products are formed.
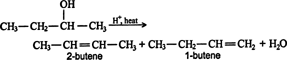
The Zaitsev rule predicts that the major product is 2-butene. Notice that each carbon atom involved in the double bond of 2-butene has one methyl group attached to it. In the case of 1-butene, one carbon atom of the double bond has one substituent (the ethyl group), while the other carbon atom has no substituents.
Add Answer to:
Draw the major product of the following alcohol dehydration in
the presence of H2SO4. Use the...
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the...
 Draw the major product of the following alcohol dehydration in
the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to
predict the major product when necessary. Include all hydrogen
atoms.
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.
Draw the major product of the following alcohol dehydration in
the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to
predict the major product when necessary. Include all hydrogen
atoms.
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.
1) Predict the following major alkene product that would result from dehydration of the following alcohols...
 1)
Predict the following major alkene product that would result from
dehydration of the following alcohols ,with no carbocation
rearrangements:
2) Draw the mechanism for the following dehydration
reaction:
** I already asked these questions, the tutor used conc.
H2SO4... Could I use H3PO4 instead? For #2, I'm not sure if I drew
the mechanism correctly can you explain and draw the mechanism
please? The pic in pencil is my attempt at #2
1)
Predict the following major alkene product that would result from
dehydration of the following alcohols ,with no carbocation
rearrangements:
2) Draw the mechanism for the following dehydration
reaction:
** I already asked these questions, the tutor used conc.
H2SO4... Could I use H3PO4 instead? For #2, I'm not sure if I drew
the mechanism correctly can you explain and draw the mechanism
please? The pic in pencil is my attempt at #2
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4...
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 + OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 + OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4...
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product. H2SO4...
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product. H2SO4 OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product. H2SO4 OH
Draw the structure of the major product of the following acid-catalyzed dehydration. CH3 H3C H2SO4 H3CH2...
 Draw the structure of the major product of the following acid-catalyzed dehydration. CH3 H3C H2SO4 H3CH2 heat ОН
Draw the structure of the major product of the following acid-catalyzed dehydration. CH3 H3C H2SO4 H3CH2 heat ОН
For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4
 For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4, then draw the structures of the minor and major products of the elimination.
For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4, then draw the structures of the minor and major products of the elimination.
3 points Save Which alcohol would be a suitable substrate for a dehydration reaction that produces...
 3 points Save Which alcohol would be a suitable substrate for a dehydration reaction that produces the alkene shown as the major product? Assume no carbocation rearrangements occur. H2SO4
3 points Save Which alcohol would be a suitable substrate for a dehydration reaction that produces the alkene shown as the major product? Assume no carbocation rearrangements occur. H2SO4
Be sure to answer all parts Report problem The following alcohol has been subjected to acid-catalyzed...
 Be sure to answer all parts Report problem The following alcohol has been subjected to acid-catalyzed dehydration and yields a mixture of two isomeric alkenes. Draw the two alkenes, the major and the minor product as predicted by the Zaitsev rule. (CH3)2CCH(CH3)2 OH Major product: draw structure Minor product: draw structure
Be sure to answer all parts Report problem The following alcohol has been subjected to acid-catalyzed dehydration and yields a mixture of two isomeric alkenes. Draw the two alkenes, the major and the minor product as predicted by the Zaitsev rule. (CH3)2CCH(CH3)2 OH Major product: draw structure Minor product: draw structure
Draw the mechanism for the following E1 dehydration reaction and show the major product. aw the...
 Draw the mechanism for the following E1 dehydration reaction
and show the major product.
aw the mechanism for the following E1 dehydration reaction and show the major product. + H2SO4 OH
Draw the mechanism for the following E1 dehydration reaction
and show the major product.
aw the mechanism for the following E1 dehydration reaction and show the major product. + H2SO4 OH
Most questions answered within 3 hours.
-
Please explain steps:
An 80 kg swimmer steps off a platform 10 m above the water...
asked 27 minutes ago -
A lottery exists where balls numbered 1 to 17 are placed in an
urn. To win,...
asked 30 minutes ago -
26) Briefly describe, using words or simple diagrams, the
chemiosmotic theory for coupling oxidation to phosphorylation...
asked 2 hours ago -
Suppose that XX is a random variable with mean 16 and standard
deviation 5 . Also...
asked 3 hours ago -
Calculate the number density of argon gas at a temperature of
24C and a pressure of...
asked 6 hours ago -
Alternative
Classification
How to Estimate
Probabilities from Data? ( For continuous Attributes)
And How to generate...
asked 6 hours ago -
An explosion breaks a 20.0-kg object into three parts. The
object is initially moving at a...
asked 7 hours ago -
Calculate the approximate number of residues of Rubisco, which
is involved in carbon fixation in plants,...
asked 8 hours ago -
Other decisions about scientific claims can have a much broader
impact.ENERGYarrow-10x10.png, environment, health, security - all...
asked 9 hours ago -
I need to write a research paper and work cited about this
topic: The United States...
asked 9 hours ago -
Hello! I was wondering if I could have some help?
If the vapor pressure of carvone...
asked 10 hours ago -
An economist wants to estimate the mean per capita income (in
thousands of dollars) for a...
asked 10 hours ago
 Draw the major product of the following alcohol dehydration in
the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to
predict the major product when necessary. Include all hydrogen
atoms.
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.
Draw the major product of the following alcohol dehydration in
the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to
predict the major product when necessary. Include all hydrogen
atoms.
Draw the major product of the following alcohol dehydration in the presence of H2SO4. Use the Zaitsev rule (no rearrangements) to predict the major product when necessary. Include all hydrogen atoms.
 1)
Predict the following major alkene product that would result from
dehydration of the following alcohols ,with no carbocation
rearrangements:
2) Draw the mechanism for the following dehydration
reaction:
** I already asked these questions, the tutor used conc.
H2SO4... Could I use H3PO4 instead? For #2, I'm not sure if I drew
the mechanism correctly can you explain and draw the mechanism
please? The pic in pencil is my attempt at #2
1)
Predict the following major alkene product that would result from
dehydration of the following alcohols ,with no carbocation
rearrangements:
2) Draw the mechanism for the following dehydration
reaction:
** I already asked these questions, the tutor used conc.
H2SO4... Could I use H3PO4 instead? For #2, I'm not sure if I drew
the mechanism correctly can you explain and draw the mechanism
please? The pic in pencil is my attempt at #2
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 + OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 + OH
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product H2SO4 OH
 5. Draw the mechanism for the following E1 dehydration reaction and show the major product. H2SO4 OH
5. Draw the mechanism for the following E1 dehydration reaction and show the major product. H2SO4 OH
 Draw the structure of the major product of the following acid-catalyzed dehydration. CH3 H3C H2SO4 H3CH2 heat ОН
Draw the structure of the major product of the following acid-catalyzed dehydration. CH3 H3C H2SO4 H3CH2 heat ОН
 For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4, then draw the structures of the minor and major products of the elimination.
For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4, then draw the structures of the minor and major products of the elimination.
 3 points Save Which alcohol would be a suitable substrate for a dehydration reaction that produces the alkene shown as the major product? Assume no carbocation rearrangements occur. H2SO4
3 points Save Which alcohol would be a suitable substrate for a dehydration reaction that produces the alkene shown as the major product? Assume no carbocation rearrangements occur. H2SO4
 Be sure to answer all parts Report problem The following alcohol has been subjected to acid-catalyzed dehydration and yields a mixture of two isomeric alkenes. Draw the two alkenes, the major and the minor product as predicted by the Zaitsev rule. (CH3)2CCH(CH3)2 OH Major product: draw structure Minor product: draw structure
Be sure to answer all parts Report problem The following alcohol has been subjected to acid-catalyzed dehydration and yields a mixture of two isomeric alkenes. Draw the two alkenes, the major and the minor product as predicted by the Zaitsev rule. (CH3)2CCH(CH3)2 OH Major product: draw structure Minor product: draw structure
 Draw the mechanism for the following E1 dehydration reaction
and show the major product.
aw the mechanism for the following E1 dehydration reaction and show the major product. + H2SO4 OH
Draw the mechanism for the following E1 dehydration reaction
and show the major product.
aw the mechanism for the following E1 dehydration reaction and show the major product. + H2SO4 OH