Homework Answers
Add Answer to:
A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in...
ed to a Carnot refrigerator so that all of the work produced by the engine is...
 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
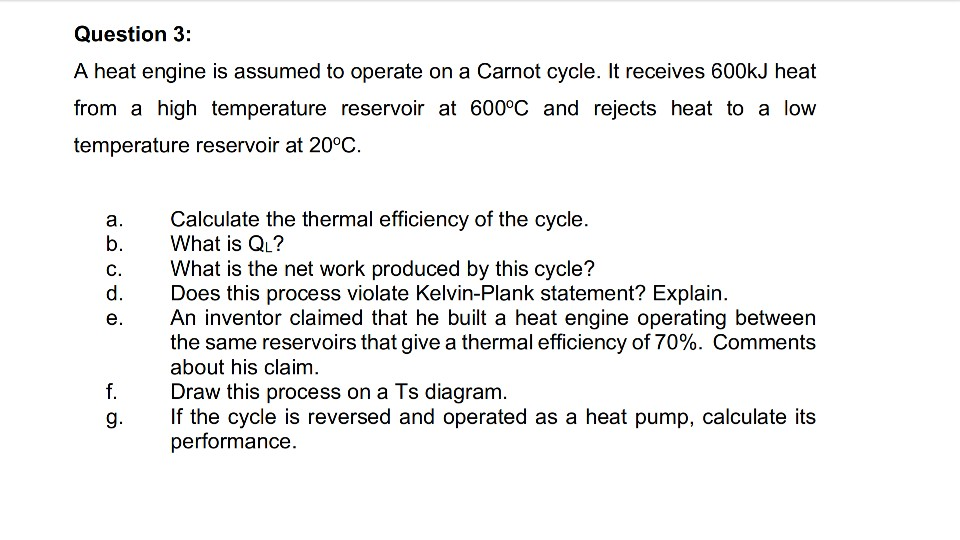 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
step by step explanation please 04. (a) A heat engine, as shown in in Figure Q4,...
 step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
Hi, please explain solution in full. Thanks in advance. Question: a) i) A Carnot engine which...
Hi, please explain solution in full. Thanks in advance. Question: a) i) A Carnot engine which operates between two heat reservoirs has an efficiency of η = 10%. [3] Calculate a coefficient of refrigeration performance of the Carnot refrigerator operates between the same reservoirs. ii)m1 = 300 g of copper with initial temperature T1 = 97 [3] ◦C is deep into 0.1 ` of water with initial temperature T2 = 7◦C. The water density is ρ = 1 kg `...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
Answer Description The Carnot cycle attempts to model the most efficient possible process by avoiding what?...
 Answer Description The Carnot cycle attempts to model the most efficient possible process by avoiding what? 1. Adiabatic processes 2. Isothermal processes 3. Irreversible processes 4. Reversible processes For the Carnot cycle, in what type of process is heat added or removed? 1. Adiabatic 2. Isobaric 3. Isochoric 4. Isothermal Which statement is true? 1. n carnot < nactual 2. n Carnot = nactual 3. n carnot > nactual D. A heat engine takes from heat from a reservoir at...
Answer Description The Carnot cycle attempts to model the most efficient possible process by avoiding what? 1. Adiabatic processes 2. Isothermal processes 3. Irreversible processes 4. Reversible processes For the Carnot cycle, in what type of process is heat added or removed? 1. Adiabatic 2. Isobaric 3. Isochoric 4. Isothermal Which statement is true? 1. n carnot < nactual 2. n Carnot = nactual 3. n carnot > nactual D. A heat engine takes from heat from a reservoir at...
A Carnot engine operates us ing 1.0 mol e of monoatomic ideal gas as a working...
A Carnot engine operates us ing 1.0 mol e of monoatomic ideal gas as a working s ubstance. In t he first step, the gas is place d in thermal contact with a heat reservoir and expands isothermally to 3 .0 times its initial volume. (a) If the internal energy o f the gas after this step is 6.25 k J , calculate the temperature of the heat reservoir ( T h ) . (b) C alculate the heat absorbed...
Problem 6. Why do you think Carnot engine is the most efficient engine? Choose all correct...
 Problem 6. Why do you think Carnot engine is the most efficient engine? Choose all correct answers. 4 marks a. adiabatic and isothermal paths are most efficient out of all paths. b. the cold reservoir is at 0 K temperature. c. the processes are done reversibly. d. as long as you do work in a cycle, the engine is always going to be efficient. e. the two adiabatic processes where there is no heat lost is the key. f. no...
Problem 6. Why do you think Carnot engine is the most efficient engine? Choose all correct answers. 4 marks a. adiabatic and isothermal paths are most efficient out of all paths. b. the cold reservoir is at 0 K temperature. c. the processes are done reversibly. d. as long as you do work in a cycle, the engine is always going to be efficient. e. the two adiabatic processes where there is no heat lost is the key. f. no...
Most questions answered within 3 hours.
-
What is the path of a carbon atom during photosynthesis , light
dependent and non dependent,...
asked 1 minute from now -
The balanced equation for the combustion of butane,
C4H10, is
2 C4H10(g) + 13
O2(g) →...
asked 1 minute from now -
Q1 At depths of 2000 m in the sea, the pressure is about 200
times atmospheric...
asked 14 minutes ago -
A value commonly taken for the perception reaction time of
motorists is delta t equals 1.5...
asked 6 minutes ago -
For
a test of independent groups, a t test can still be conducted even
when assumptions...
asked 27 minutes ago -
What assumption are we making about ratings e.g., that ratings
are important in motivating poor performers...
asked 34 minutes ago -
You’ve observed the following returns on Crash-n-Burn Computer’s
stock over the past five years: 11 percent,...
asked 36 minutes ago -
In which of the following cases is the displacement's
magnitude half the distance traveled? a. 10...
asked 57 minutes ago -
(InputMismatchException) and (ArrayIndexOutOfBoundsException):
Using the two arrays shown below, write a program that prompts the
user...
asked 52 minutes ago -
A basketball of mass m = 630 g rolls off the hoop's rim, falls
from a...
asked 1 hour ago -
What is the activity [A] of Na+ in a .05M solution of NaCl at
25C? You...
asked 1 hour ago -
You own a bond portfolio worth $41,000. You estimate that your
portfolio has an average YTM...
asked 1 hour ago


 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
 step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
 Answer Description The Carnot cycle attempts to model the most efficient possible process by avoiding what? 1. Adiabatic processes 2. Isothermal processes 3. Irreversible processes 4. Reversible processes For the Carnot cycle, in what type of process is heat added or removed? 1. Adiabatic 2. Isobaric 3. Isochoric 4. Isothermal Which statement is true? 1. n carnot < nactual 2. n Carnot = nactual 3. n carnot > nactual D. A heat engine takes from heat from a reservoir at...
Answer Description The Carnot cycle attempts to model the most efficient possible process by avoiding what? 1. Adiabatic processes 2. Isothermal processes 3. Irreversible processes 4. Reversible processes For the Carnot cycle, in what type of process is heat added or removed? 1. Adiabatic 2. Isobaric 3. Isochoric 4. Isothermal Which statement is true? 1. n carnot < nactual 2. n Carnot = nactual 3. n carnot > nactual D. A heat engine takes from heat from a reservoir at...
 Problem 6. Why do you think Carnot engine is the most efficient engine? Choose all correct answers. 4 marks a. adiabatic and isothermal paths are most efficient out of all paths. b. the cold reservoir is at 0 K temperature. c. the processes are done reversibly. d. as long as you do work in a cycle, the engine is always going to be efficient. e. the two adiabatic processes where there is no heat lost is the key. f. no...
Problem 6. Why do you think Carnot engine is the most efficient engine? Choose all correct answers. 4 marks a. adiabatic and isothermal paths are most efficient out of all paths. b. the cold reservoir is at 0 K temperature. c. the processes are done reversibly. d. as long as you do work in a cycle, the engine is always going to be efficient. e. the two adiabatic processes where there is no heat lost is the key. f. no...