Homework Answers
Q = 370 J
Th = 145 deg C ; eta = 24%
D)The netropy of the world changes by "zero"
delta S = 0 J/K
Since, Carnot cycle has reversible process.
E)we need to find W for this.
eta = W/Q
W = eta x Q = 0.24 x 370 = 88.8 J
PE = W
m g h = 88.8
m = 88.8/g h = 88.8/9.8 x 25 = 0.362 kg
Hence, m = 0.362 kg.
Add Answer to:
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle...
A heat engine operates in a Carnot cycle between 86.0°C and 345°C. It absorbs 20,000 J...
A heat engine operates in a Carnot cycle between 86.0°C and 345°C. It absorbs 20,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 5.00 s. (a) What is the mechanical power output of this engine? (b) How much energy does it expel in each cycle by heat?
1.The efficiency of a Carnot engine is 27%. The engine absorbs 826 J of energy per...
1.The efficiency of a Carnot engine is 27%. The engine absorbs 826 J of energy per cycle by heat from a hot reservoir at 503 K. (a) Determine the energy expelled per cycle. _ J (b) Determine the temperature of the cold reservoir. _K 2.A sample of helium behaves as an ideal gas as energy is added by heat at constant pressure from 273 K to 343 K. If 15.0 J of work is done by the gas during this...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
During each cycle, a heat engine operating between two heat reservoirs absorbs 156 J from the...
During each cycle, a heat engine operating between two heat reservoirs absorbs 156 J from the reservoir at 100°C and releases 136 J to the reservoir at 20°C. (a) What is the efficiency of this engine? % (b) What is the ratio of its efficiency to that of a Carnot engine working between the same reservoirs? This ratio is called the second law efficiency. εengine / εCarnot =
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310...
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310 J to a low-temperature reservoir. If the efficiency of this engine is 59% of the efficiency of a Carnot engine, what is the ratio of the low temperature to the high temperature in the Carnot engine?
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
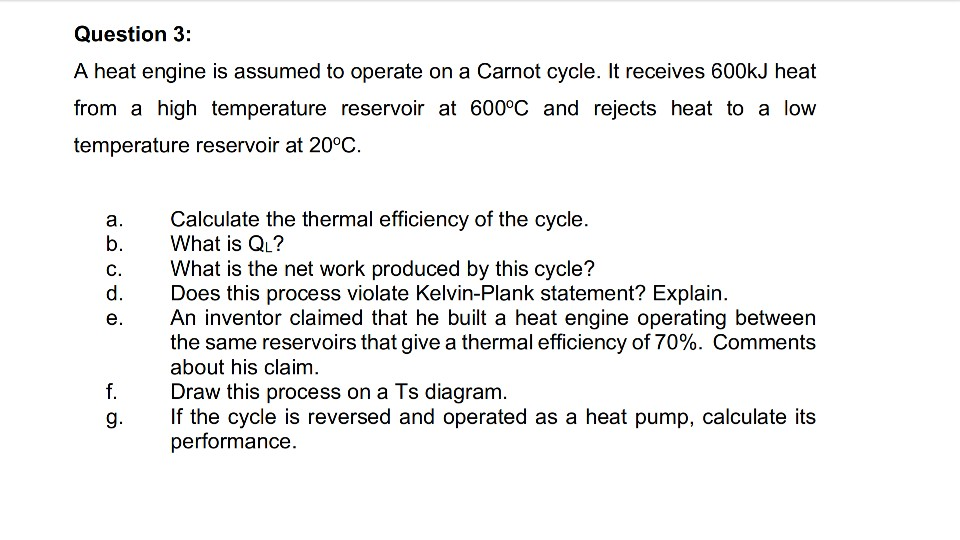 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J...
 A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
Most questions answered within 3 hours.
-
Calculate the volume in milliliters of a 1.4 mol/L barium
acetate solution that contains 200.g of...
asked 2 minutes from now -
A consumer can choose between two gambles. The “sure thing”
guaranteesadditional income (I) of $250,000. The...
asked 1 minute from now -
There are 9 women and 6 men in a department. A committee of four
is to...
asked 50 seconds ago -
Arthur Meiners is the production manager of Wheel-Rite, a small
producer of metal parts. Wheel-Rite supplies...
asked 15 minutes ago -
Company Risk Premium A company has a beta of
4.57. If the market return is expected...
asked 14 minutes ago -
3. Which statement about nuclear fission is correct? (1
point)
A. Nuclear fission provides energy for...
asked 20 minutes ago -
If a $2,000 increase in income leads to a $1,5000 increase in
consumption expenditures, then the...
asked 19 minutes ago -
May you please put this in layman's terms?
ABSTRACT
Coagulase-negative staphylococci (CoNS) and Staphylococcus
aureus are...
asked 24 minutes ago -
If authentic leadership is really a lifelong process,
can teenagers be authentic leaders? Why or why...
asked 40 minutes ago -
Six years of quarterly data of a seasonally adjusted series are
used to estimate a linear...
asked 58 minutes ago -
Which of the following is not an ecological model used
to foster behavior change?
PRECEDE-PROCEED Model...
asked 1 hour ago -
On the Apollo 14 mission to the moon, astronaut Alan Shepard hit
a golf ball with...
asked 58 minutes ago

 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
 A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW