Homework Answers

Add Answer to:
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
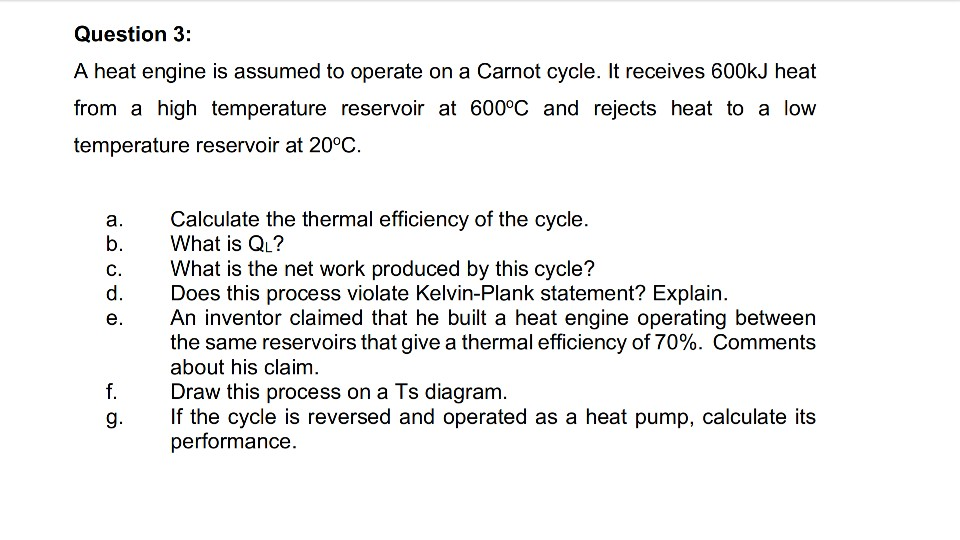 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Problem 1 The work output (1000 kJ) and thermal efficiency of a Carnot heat engine (50%)...
 Problem 1 The work output (1000 kJ) and thermal efficiency of a Carnot heat engine (50%) are given. The heat supplied to the heat engine, the heat rejected and the temperature of heat sink are to be determined 1200 C Qn 5096 HE1000 kJ sink
Problem 1 The work output (1000 kJ) and thermal efficiency of a Carnot heat engine (50%) are given. The heat supplied to the heat engine, the heat rejected and the temperature of heat sink are to be determined 1200 C Qn 5096 HE1000 kJ sink
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C...
 Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C. 900 "C 800 kI min Determine: 27"C (a) The Camot heat engine thermal efficiency...
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C. 900 "C 800 kI min Determine: 27"C (a) The Camot heat engine thermal efficiency...
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C...
 Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C 900 ℃ 800 kI min Determine: 27-C (a) The Camot heat engine thermal efficiency...
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C 900 ℃ 800 kI min Determine: 27-C (a) The Camot heat engine thermal efficiency...
step by step explanation please 04. (a) A heat engine, as shown in in Figure Q4,...
 step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
In one cycle, a certain heat engine takes in 1000 J1000 J of heat from its...
 In one cycle, a certain heat engine takes in 1000 J1000 J of
heat from its high‑temperature reservoir and exhausts 700 J700 J of
heat to its low‑temperature reservoir.
What is the efficiency of the heat engine under these
conditions? 70%,30%,43%,143%,300%.
In one cycle, a certain heat engine takes in 1000 J of heat from its high-temperature reservoir and exhausts 700 J of heat to its low-temperature reservoir. Hot reservoir What is the efficiency of the heat engine under these...
In one cycle, a certain heat engine takes in 1000 J1000 J of
heat from its high‑temperature reservoir and exhausts 700 J700 J of
heat to its low‑temperature reservoir.
What is the efficiency of the heat engine under these
conditions? 70%,30%,43%,143%,300%.
In one cycle, a certain heat engine takes in 1000 J of heat from its high-temperature reservoir and exhausts 700 J of heat to its low-temperature reservoir. Hot reservoir What is the efficiency of the heat engine under these...
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310...
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310 J to a low-temperature reservoir. If the efficiency of this engine is 59% of the efficiency of a Carnot engine, what is the ratio of the low temperature to the high temperature in the Carnot engine?
Most questions answered within 3 hours.
-
Suppose we have a binomial experiment in which success is
defined to be a particular quality...
asked 14 minutes ago -
march the type of cellular control with the description: enzyme
induction and the enzyme repression. How...
asked 24 minutes ago -
Brief Exercise 5-09 (Part Level Submission)
The following information relates to Blue Spruce Corp. for the...
asked 24 minutes ago -
An unknown amount of a compound with a molecular mass of 284.04
g/mol is dissolved in...
asked 29 minutes ago -
You are at rest at a stop sign. There is another stop sign that
is 100...
asked 30 minutes ago -
Calculate the equilibrium electrode potential for Fe3+/Fe2+
redox system, if the initial concentration of Fe2+ is...
asked 34 minutes ago -
Describe in detail with graph and diagram how bonding force,
bonding curves and bonding energy at...
asked 51 minutes ago -
Gingerbread cookies become inedible if not eaten quickly enough.
Clarence is trying to determine how many...
asked 46 minutes ago -
A
crane lifts a 200 kg block a height of 10 m in 18 seconds. What...
asked 49 minutes ago -
A 12.0-g bullet is fired horizontally into a 115-g wooden block
that is initially at rest...
asked 49 minutes ago -
Which DNA primer would have the HIGHEST melting temperature?
Question 17 options:
a)
GCATCGGC
b)
AATCGGAT...
asked 57 minutes ago -
what is the charge on the chromium ion in Cr2O3.
a -3
b -2
c 0...
asked 57 minutes ago

 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
 Problem 1 The work output (1000 kJ) and thermal efficiency of a Carnot heat engine (50%) are given. The heat supplied to the heat engine, the heat rejected and the temperature of heat sink are to be determined 1200 C Qn 5096 HE1000 kJ sink
Problem 1 The work output (1000 kJ) and thermal efficiency of a Carnot heat engine (50%) are given. The heat supplied to the heat engine, the heat rejected and the temperature of heat sink are to be determined 1200 C Qn 5096 HE1000 kJ sink
 Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C. 900 "C 800 kI min Determine: 27"C (a) The Camot heat engine thermal efficiency...
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C. 900 "C 800 kI min Determine: 27"C (a) The Camot heat engine thermal efficiency...
 Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C 900 ℃ 800 kI min Determine: 27-C (a) The Camot heat engine thermal efficiency...
Page 2 of4 Problem A Carnot heat engine received heat from a reservoir at 900 °C at a rate of 800 k./min and reject the waste heat to the sink at 27°C. The entire work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the refrigerated space at -5 *C and transfer it to the same sink at 27°C 900 ℃ 800 kI min Determine: 27-C (a) The Camot heat engine thermal efficiency...
 step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
step by step explanation please
04. (a) A heat engine, as shown in in Figure Q4, is operating on a Carnot cycle and has a thermal efficiency of 65%. The waste heat from this engine is rejected to a nearby lake with a temperature of 10°C at a rate of 900 kJ/min High Temperature Reservoir Q, Carnot Heat Engine a =9(0 kJ/min Low Temperature Reservoir T 10°C Figure Q4. Carnot heat engine in kW (i) Determine the net power output...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
 In one cycle, a certain heat engine takes in 1000 J1000 J of
heat from its high‑temperature reservoir and exhausts 700 J700 J of
heat to its low‑temperature reservoir.
What is the efficiency of the heat engine under these
conditions? 70%,30%,43%,143%,300%.
In one cycle, a certain heat engine takes in 1000 J of heat from its high-temperature reservoir and exhausts 700 J of heat to its low-temperature reservoir. Hot reservoir What is the efficiency of the heat engine under these...
In one cycle, a certain heat engine takes in 1000 J1000 J of
heat from its high‑temperature reservoir and exhausts 700 J700 J of
heat to its low‑temperature reservoir.
What is the efficiency of the heat engine under these
conditions? 70%,30%,43%,143%,300%.
In one cycle, a certain heat engine takes in 1000 J of heat from its high-temperature reservoir and exhausts 700 J of heat to its low-temperature reservoir. Hot reservoir What is the efficiency of the heat engine under these...