Homework Answers


Add Answer to:
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
Operating in series are two reversible heat pumps. Heat transfer gives energy to the first cycle...
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
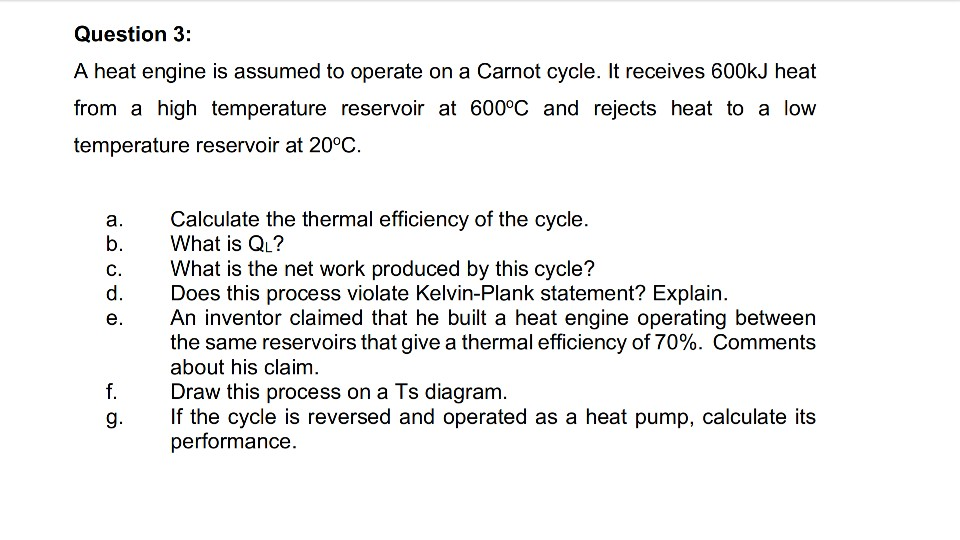 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
A Carnot engine is operated between two heat reservoirs at temperatures of 400 K and 300...
 A Carnot engine is operated between two heat reservoirs at temperatures of 400 K and 300 K. (a) If the engine receives 5 x 103 kJ from the reservoir at 400 K in each cycle, how many joules does it reject to the reservoir at 300 K? (b) If the engine is operated as a refrigerator (ie., in reverse) and receives 5 × 103 kJ from the reservoir at 300 K, how many joules does it deliver to the reservoir...
A Carnot engine is operated between two heat reservoirs at temperatures of 400 K and 300 K. (a) If the engine receives 5 x 103 kJ from the reservoir at 400 K in each cycle, how many joules does it reject to the reservoir at 300 K? (b) If the engine is operated as a refrigerator (ie., in reverse) and receives 5 × 103 kJ from the reservoir at 300 K, how many joules does it deliver to the reservoir...
A power cycle operating between two thermal reservoirs receives energy QH by heat transfer from a...
A power cycle operating between two thermal reservoirs receives energy QH by heat transfer from a hot reservoir at TH = 2000 K and rejects energy QC by heat transfer to a cold reservoir at TC = 400 K. For each of the following cases determine whether the cycle operates reversibly, operates irreversibly, or is impossible. (a) QH = 1000 kJ, ƞ = 60% (b) QH = 1000 kJ, Wcycle = 850 kJ (c) QH = 1000 kJ, QC =...
As shown in the figure, a reversible power cycle receives energy QH by heat transfer from...
 As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K)...
 8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
1. A Carnot cycle receives 1000 kJ of heat at 800 ºC and rejects heat at...
1. A Carnot cycle receives 1000 kJ of heat at 800 ºC and rejects heat at 300 ºC. Calculate the work output. 2. Steam enters an adiabatic turbine at 7MPa and 600 ◦C and leaves at a pressure of 400 kPa. Determine the maximum amount of work that can be delivered by the turbine.
a) Assume that the total cost of operation of a heat pump is four times the...
a) Assume that the total cost of operation of a heat pump is four times the theoretical power cost for perfect efficiency, whereas the cost of direct electrical heating is just the power cost. If it is required to maintain room temperature at 27 °C, what limitations on the outside temperature will be required to economically justify the use of heat pump? (b) A Carnot heat engine with an efficiency of 75% receives heat from a source at a rate...
Most questions answered within 3 hours.
-
lease solve all the
questions, don't need to explanations
Q1 - All animal
species have general...
asked 1 hour ago -
Business Phasing
1.Discuss the logical progression for growing a business, which
starts from the initial idea...
asked 1 hour ago -
Modify
When executing on the command line having only
this program name, the program will accept...
asked 2 hours ago -
Kenny Electric Company's noncallable bonds were issued several
years ago and now have 20 years to...
asked 3 hours ago -
find H(e^Jtheta) at theta= 0, pi/10, pi/20, pi/2 for
the following:
a) H(e^Jtheta)= 1+e^Jtheta
b) H(e^Jtheta)=...
asked 3 hours ago -
Home Corporation will open a new store on January 1. Based on
experience from its other...
asked 3 hours ago -
In a neoclassical model, use the IS-LM to analyze the effect of
a permanent money supply...
asked 4 hours ago -
An electron passes through a point 2.67 cm from a long straight
wire as it moves...
asked 5 hours ago -
A grammar is a 4-tuple G, G = (Ν, Σ, Π, Σ, S) where, Ν is...
asked 5 hours ago -
In this part, calculate the present values. Use the Excel PV
function to compute the present...
asked 5 hours ago -
Part 1. Primitive Types, Sorting, Recursion for
Homework.java
a) Implement the static method initializeArray that receives...
asked 6 hours ago -
Using C++, build a sorter that can rank a sequence of numbers in
a descending order....
asked 6 hours ago

 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
 A Carnot engine is operated between two heat reservoirs at temperatures of 400 K and 300 K. (a) If the engine receives 5 x 103 kJ from the reservoir at 400 K in each cycle, how many joules does it reject to the reservoir at 300 K? (b) If the engine is operated as a refrigerator (ie., in reverse) and receives 5 × 103 kJ from the reservoir at 300 K, how many joules does it deliver to the reservoir...
A Carnot engine is operated between two heat reservoirs at temperatures of 400 K and 300 K. (a) If the engine receives 5 x 103 kJ from the reservoir at 400 K in each cycle, how many joules does it reject to the reservoir at 300 K? (b) If the engine is operated as a refrigerator (ie., in reverse) and receives 5 × 103 kJ from the reservoir at 300 K, how many joules does it deliver to the reservoir...
 As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
 8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.