1. A Carnot cycle receives 1000 kJ of heat at 800 ºC and rejects heat at...
1. A Carnot cycle receives 1000 kJ of heat at 800 ºC and rejects heat at 300 ºC. Calculate the work output.
2. Steam enters an adiabatic turbine at 7MPa and 600 ◦C and leaves at a pressure of 400 kPa. Determine the maximum amount of work that can be delivered by the turbine.
Homework Answers

Add Answer to:
1. A Carnot cycle receives 1000 kJ of heat at 800 ºC and rejects
heat at...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a...
 Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal efficiency of...
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal efficiency of...
1. Consider a Carnot cycle where Th=1000. K and Tc=300. K. The heat absorbed, qh, is...
1. Consider a Carnot cycle where Th=1000. K and Tc=300. K. The heat absorbed, qh, is 200. kJ. What is the entropy change for the entire cycle? 2. Consider a Carnot cycle where Th=1000. K and Tc=300. K. The heat absorbed, qh, is 200. kJ. What is the entropy change for the adiabatic expansion? 3. Consider a Carnot cycle where Th=1000. K and Tc=300. K. The heat absorbed, qh, is 200. kJ. What is the entropy change during the isothermal...
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive...
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive a Carnot refrigerator that removes heat from -15oC space at a rate of 400kJ/min and rejects the heat to 25 oC environment. Find the rate of heat supplied to the heat engine Find the total rate of heat rejection to the environment ii) In the steady state of a steam turbine operation, steam enters a turbine at a rate of 25000 kg/hr at 4MPa...
Example A steam turbine receives steam with a specific enthalpy of 3121 kJ/kg. The steam leaves...
 Example A steam turbine receives steam with a specific enthalpy of 3121 kJ/kg. The steam leaves the turbine with a specific enthalpy of 2676 kJ/kg. The steam enters and leaves the turbine with velocities of 15 m/s and 60m/s. The elevation difference between the entry and exit ports is negligible and the heat energy lost through the turbine walls is 7600 kJ/h. Calculate the power output if the mass flow through the turbine is 0.5 kg/s. High pressure steam Low...
Example A steam turbine receives steam with a specific enthalpy of 3121 kJ/kg. The steam leaves the turbine with a specific enthalpy of 2676 kJ/kg. The steam enters and leaves the turbine with velocities of 15 m/s and 60m/s. The elevation difference between the entry and exit ports is negligible and the heat energy lost through the turbine walls is 7600 kJ/h. Calculate the power output if the mass flow through the turbine is 0.5 kg/s. High pressure steam Low...
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
Tutorial Questions 1 1. Water is the working fluid in an ideal Rankine cycle. The condenser...
Tutorial Questions 1.1. Water is the working fluid in an ideal Rankine cycle. The condenser pressure is kPa, and saturated vapor enters the turbine at 10 MPa. Determine the heat transfer rates, in kJ per kg of steam flowing, for the working fluid passing through the boiler and condenser and calculate the thermal efficiency.2. Water is the working fluid in an ideal Rankine cycle. Saturated vapor enters the turbine at 16 MPa, and the condenser pressure is 8 kPa ....
PROBLEM 2 Problem 1 (40 pts): A power plant runs on a steam cycle that closely...
 PROBLEM 2
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal...
PROBLEM 2
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
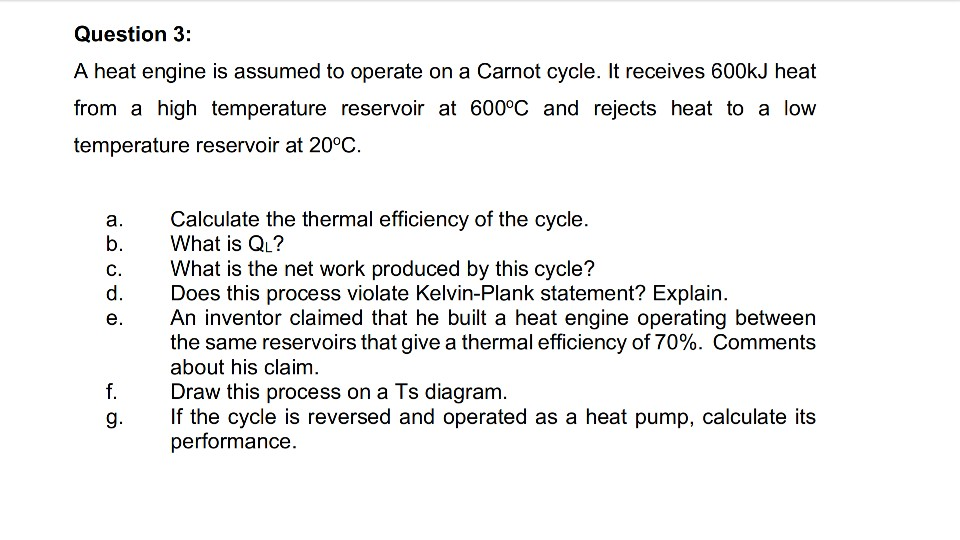 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 5 MPa and 500°C and is condensed in the condenser at a pressure of 50 kPa. Heat is supplied to th...
 Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 5 MPa and 500°C and is condensed in the condenser at a pressure of 50 kPa. Heat is supplied to the steam in a furnace maintained at 800 K, and waste heat is rejected to the surroundings at 300 K. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the net work output, (b) the thermal efficiency...
Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 5 MPa and 500°C and is condensed in the condenser at a pressure of 50 kPa. Heat is supplied to the steam in a furnace maintained at 800 K, and waste heat is rejected to the surroundings at 300 K. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the net work output, (b) the thermal efficiency...
Most questions answered within 3 hours.
-
In JAVA please (need answers in a few hours!)
Exercise #2: Design and implement a program...
asked 2 hours ago -
The mass spectrum of an organic compound shows the relative
abundances of M to be 53.76%...
asked 3 hours ago -
Coca Cola’s strategy of “think local, act local” represents a
__________ approach.
Question options:
1)
transnational...
asked 4 hours ago -
which of the following is not a category of project management
risk?
a) external
b) internal...
asked 4 hours ago -
Focus on Critical Thinking: Are citizen suit provisions an
effective way to achieve environmental objectives? Do...
asked 5 hours ago -
Gaseous butane CH3CH22CH3 will react with gaseous oxygen O2 to
produce gaseous carbon dioxide CO2 and...
asked 5 hours ago -
Required to construct counters using synchronous sequential
logic. Use one hex digit to display the result....
asked 5 hours ago -
(Ultra) Large-Scale Systems –Characteristics?
explain in detail
How the nature of an enterprise affect complex...
asked 6 hours ago -
Some of the antibiotic susceptible strains show colonies within
the clear zone. What it does this...
asked 6 hours ago -
In the lottery game Fantasy 5 you have to select 5 numbers from
the numbers {1,2,3,.......,38,39}....
asked 6 hours ago -
A call option on Jupiter Motors stock with an exercise price of
$80 and one-year expiration...
asked 6 hours ago -
What is the disadvantage of an automated vulnerability scan tool
like Nessus?
prone to false negatives...
asked 6 hours ago
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
 Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal efficiency of...
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal efficiency of...
 Example A steam turbine receives steam with a specific enthalpy of 3121 kJ/kg. The steam leaves the turbine with a specific enthalpy of 2676 kJ/kg. The steam enters and leaves the turbine with velocities of 15 m/s and 60m/s. The elevation difference between the entry and exit ports is negligible and the heat energy lost through the turbine walls is 7600 kJ/h. Calculate the power output if the mass flow through the turbine is 0.5 kg/s. High pressure steam Low...
Example A steam turbine receives steam with a specific enthalpy of 3121 kJ/kg. The steam leaves the turbine with a specific enthalpy of 2676 kJ/kg. The steam enters and leaves the turbine with velocities of 15 m/s and 60m/s. The elevation difference between the entry and exit ports is negligible and the heat energy lost through the turbine walls is 7600 kJ/h. Calculate the power output if the mass flow through the turbine is 0.5 kg/s. High pressure steam Low...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
 PROBLEM 2
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal...
PROBLEM 2
Problem 1 (40 pts): A power plant runs on a steam cycle that closely approximates a Rankine cycle with one exception: the turbine is adiabatic but not isentropic, and has an isentropic efficiency of 85%. The cycle operates between 20 kPa and 20 MPa and steam achieves a maximum temperature of 600 °C. The cycle receives heat from a combustion chamber at 1500 K and rejects heat to the surrounding. a) Calculate the net work output and thermal...
 Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 5 MPa and 500°C and is condensed in the condenser at a pressure of 50 kPa. Heat is supplied to the steam in a furnace maintained at 800 K, and waste heat is rejected to the surroundings at 300 K. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the net work output, (b) the thermal efficiency...
Consider a steam power plant operating on the simple ideal Rankine cycle. Steam enters the turbine at 5 MPa and 500°C and is condensed in the condenser at a pressure of 50 kPa. Heat is supplied to the steam in a furnace maintained at 800 K, and waste heat is rejected to the surroundings at 300 K. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the net work output, (b) the thermal efficiency...