Homework Answers
1)
carnot efficiency = 1 - Tsink / Tsource = 1 - 300/2000 = 85%
Work output / Power input = 85%
work output = 0.85 x 500kW
= 425 kW
2) actual work output = 300kW
actual efficiency = 300 /500 = 60%
3)enegy loss = theoretical work output - actual work output = 425 - 300 = 125kW
Add Answer to:
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of...
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
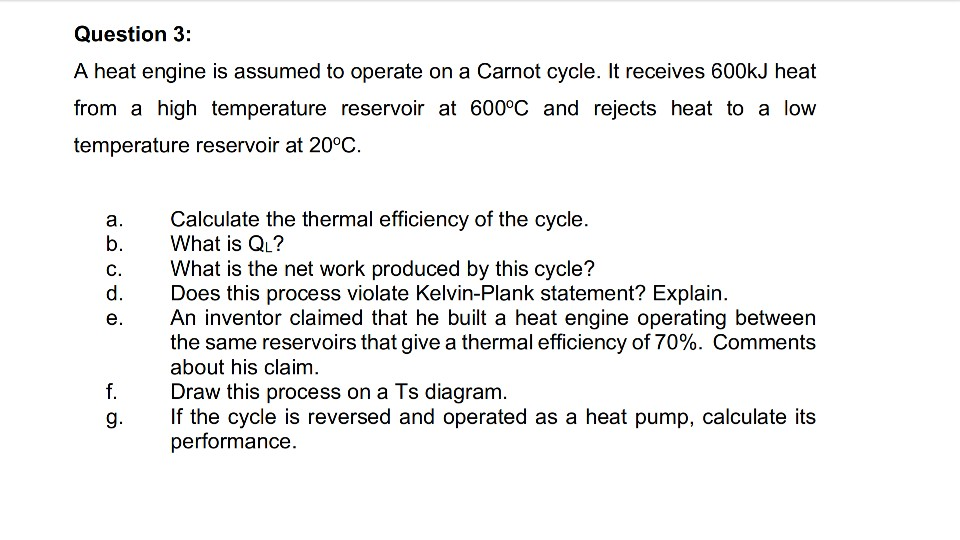 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at...
 (12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J...
 A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
A reversible heat engine receives heat of 2000 kJ from a furnace at temperature of 600...
A reversible heat engine receives heat of 2000 kJ from a furnace at temperature of 600 0C and rejects waste heat into the house. The portion of work produced by this heat engine utilized to drive a reversible heat pump to warmup the same house during the winter. The house is to be maintained at 21 0C at all times even though outside temperature drops to -15 0C. If the net-work output of the combined heat engine and heat pump...
Heat engine of Carnot cycle gained heat at 850 K and dissipated the heat to the...
Heat engine of Carnot cycle gained heat at 850 K and dissipated the heat to the outdoor at 330 K. The overall work output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cooled space at (- 22) °C at a rate of 520 kJ/min and rejects it to the same environment at 420 K. Determine: (a) the rate of heat supplied to the heat engine. (b) the total rate of heat rejection...
4. A refrigeration unit operates in an inverted Carnot cycle. It receives from the surroundings (at...
 4. A refrigeration unit operates in an inverted Carnot cycle. It receives from the surroundings (at 27°C) 10,000 kcal per hour. If the temperature inside the unit must be kept at -50°C, what is the power of the unit's engine (recall that power is P = W/t)? A Carnot engine is an idealized, reversible engine. In real life, it is found that you need to use an engine capable of producing 5.50 kW to run this unit. Express the efficiency...
4. A refrigeration unit operates in an inverted Carnot cycle. It receives from the surroundings (at 27°C) 10,000 kcal per hour. If the temperature inside the unit must be kept at -50°C, what is the power of the unit's engine (recall that power is P = W/t)? A Carnot engine is an idealized, reversible engine. In real life, it is found that you need to use an engine capable of producing 5.50 kW to run this unit. Express the efficiency...
a) Assume that the total cost of operation of a heat pump is four times the...
a) Assume that the total cost of operation of a heat pump is four times the theoretical power cost for perfect efficiency, whereas the cost of direct electrical heating is just the power cost. If it is required to maintain room temperature at 27 °C, what limitations on the outside temperature will be required to economically justify the use of heat pump? (b) A Carnot heat engine with an efficiency of 75% receives heat from a source at a rate...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
Most questions answered within 3 hours.
-
Give a unary language (using only input alphabet ∑={1} )that is
not Turing- recognizable and prove...
asked 5 minutes ago -
1. Write the balanced net reaction for a Ti (s) | TiCl2 (aq) ||
InCl3 (aq)...
asked 2 minutes ago -
3) Answer the following questions:please respond in one
paragraph
In your opinion, what are the crucial...
asked 9 minutes ago -
Assume that the readings at freezing on a batch of thermometers
are normally distributed with a...
asked 14 minutes ago -
MENSA Int'l just paid a dividend of $1.25. Their growth rate in
dividends is 10% and...
asked 13 minutes ago -
4. The excitation of a 414 V, 3-phase, delta-connected
synchronous motor is such that that the...
asked 30 minutes ago -
3. (20 pts) In the Carnot engine (refer to the figure in
question 2), an ideal...
asked 37 minutes ago -
A contestant is facing 10 closed doors. Behind one of these
doors there is a prize....
asked 38 minutes ago -
Showing appropriate work and equations, what is the yield to
maturity on a share of Six...
asked 54 minutes ago -
what would be the best entry mode in shipping from the U.S to Spain
by boat...
asked 58 minutes ago -
Under certain conditions, the substance ammonium
nitrate can be broken down to form dinitrogen
monoxide and...
asked 59 minutes ago -
1. A boy stands on one end of a boat, and then walks to the
other...
asked 1 hour ago

 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
 (12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
 A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
A heat engine operates in a Carnot cycle between 80°C and 350°C. It receives 21,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 1.00 s. What is the mechanical power of this engine? Select one: a. 21 kw b. 910 kW c. 9.10 kW d. 120 kW
 4. A refrigeration unit operates in an inverted Carnot cycle. It receives from the surroundings (at 27°C) 10,000 kcal per hour. If the temperature inside the unit must be kept at -50°C, what is the power of the unit's engine (recall that power is P = W/t)? A Carnot engine is an idealized, reversible engine. In real life, it is found that you need to use an engine capable of producing 5.50 kW to run this unit. Express the efficiency...
4. A refrigeration unit operates in an inverted Carnot cycle. It receives from the surroundings (at 27°C) 10,000 kcal per hour. If the temperature inside the unit must be kept at -50°C, what is the power of the unit's engine (recall that power is P = W/t)? A Carnot engine is an idealized, reversible engine. In real life, it is found that you need to use an engine capable of producing 5.50 kW to run this unit. Express the efficiency...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...