Homework Answers
3)
given
Q_in = 150 J
temperature of hot reservoir, Th = 135 C = 135 + 273 = 408 K
efficiency, n = 225 = 0.22
a) we know, n = Wordkone/Q_in
Workdone = n*Q_in
W = 0.22*150
= 33 J <<<<<<<--------------------Answer
b) Q_out = Q_in - W
= 150 - 33
= 117 J <<<<<<<--------------------Answer
c) let Tc is the temperature of the low-temperature reservoir
now use, n = 1 - Tc/Th
Tc/Th = 1 - n
Tc = (1 - n)*Th
= (1 - 0.22)*408
= 318.2 K
= 318.2 - 273
= 45.2 C
d) number of cycles, N = Energy required to lift/Work done
by engine per cycle
= m*g*h/W
= 500*9.8*100/33
= 14848
e) Carnot engine is an ideal heat engine.
which is made up of perfect conductors and perfect insulator.
As we do not have ideal conductors and ideal insulators, we can not built carnot engine.
carnot is an ideal and hypothetical engine which can not be built practically.
Add Answer to:
3. An ideal Carnot engine has an input of 150 J of heat per cycle at...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
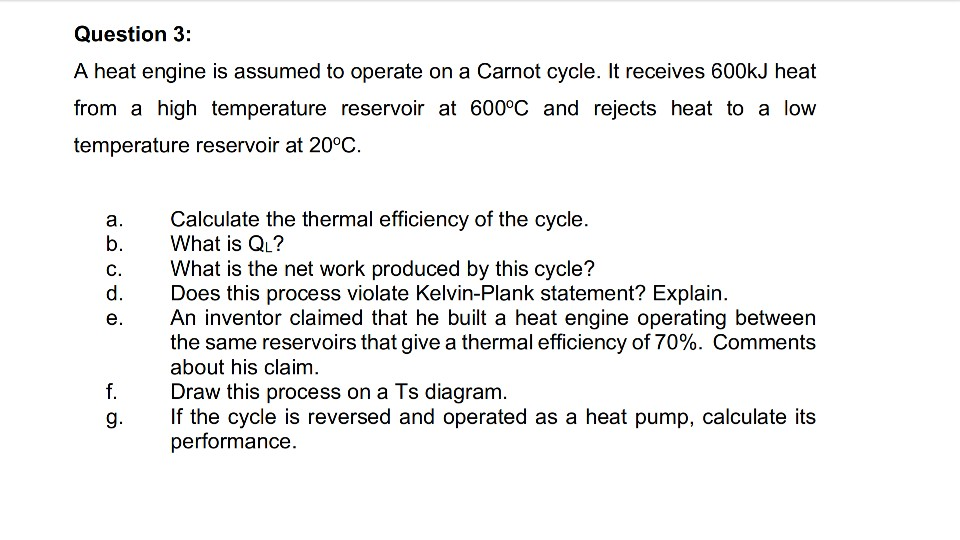 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
Problem 14-Carnot engine (14 points) The Carnot engine takes 1800 J of heat from a reservoir...
 Problem 14-Carnot engine (14 points) The Carnot engine takes 1800 J of heat from a reservoir at 600 K, does some work, and discards some heat to a reservoir at 300 K. How much work does it do, how much heat is discarded, and what is its efficiency?
Problem 14-Carnot engine (14 points) The Carnot engine takes 1800 J of heat from a reservoir at 600 K, does some work, and discards some heat to a reservoir at 300 K. How much work does it do, how much heat is discarded, and what is its efficiency?
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308 K. (a) If the engine receives 5113 J of heat from the reservoir at T1 in each cycle, how many joules per cycle does it deliver to the reservoir at T2? (b) How much mechanical work is performed by the engine during each cycle? (c) What is the thermal efficiency of the engine?
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310...
In one cycle, a heat engine absorbs 520 J from a high-temperature reservoir and expels 310 J to a low-temperature reservoir. If the efficiency of this engine is 59% of the efficiency of a Carnot engine, what is the ratio of the low temperature to the high temperature in the Carnot engine?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
Most questions answered within 3 hours.
-
Suppose you work for Allstate, and insurance company and the
data shows the following for auto...
asked 1 minute from now -
The fresh fruit market and frozen dinner market are
currently in equilibrium. Fresh fruit is a...
asked 3 minutes ago -
(1 point) Golf-course designers have become concerned that old
courses are becoming obsolete since new technology...
asked 1 minute ago -
The active retelling of dominant cultural stories is known
preserves which concept?
hegemony
heterodoxy
doxa
myth
asked 5 minutes ago -
1. Which of the following statements is true of money?
It is anything accepted in exchange...
asked 5 minutes ago -
BASED ON THE AIRBNB, ETSY, UBER: GROWING FROM ONE THOUSAND TO
ONE MILLION CUSTOMERS HARVARD CASE:...
asked 31 minutes ago -
Which of these methods cause sterilization?
Autoclaving
Dry heat
Gamma irradiation
Ethylene oxide
A.
Yes
No...
asked 37 minutes ago -
A biologist wishes to estimate the effect of an antibiotic on
the growth of a particular...
asked 45 minutes ago -
O’Deesha Company has the following information available:
Quality engineering of
products
$20,000
Quality training of
employees &n
asked 52 minutes ago -
a) Draw two water molecules.
b) Clearly name and label the type of bond that exists...
asked 2 hours ago -
C - Language
Write a loop that sets each array element to the sum of itself...
asked 3 hours ago -
(63
#14)
which of the following statments best describes how chamging
the concentration of the substances...
asked 6 hours ago

 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
 245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
245 E00 4) A Carnot heat engine loses 1000 kJ of heat per cycle to a low-temperature sink at 27 C and receives heat from a high- temperature reservoir at 927C. Determine (a) the thermal efficiency of this Carnot engine and (b) the amount of heat received from the high temperature reservoir. ఉది
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
 Problem 14-Carnot engine (14 points) The Carnot engine takes 1800 J of heat from a reservoir at 600 K, does some work, and discards some heat to a reservoir at 300 K. How much work does it do, how much heat is discarded, and what is its efficiency?
Problem 14-Carnot engine (14 points) The Carnot engine takes 1800 J of heat from a reservoir at 600 K, does some work, and discards some heat to a reservoir at 300 K. How much work does it do, how much heat is discarded, and what is its efficiency?
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...