introduction to the thermodynamics of materials
A reversible heat engine, operating in a cycle, withdraws thermal energy from a high temperature reservoir (the temperature of which consequently decreases), performs work w, and rejects thermal energy into a low-temperature reservoir (the temperature of which consequently increases). The two reservoirs are, initially, at the temperatures T1 and T2 and have constant heat capacities C1 and C2, respectively. Calculate the final temperature of the system and the maximum amount of work which can be obtained from the engine.
Homework Answers

8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K)...
 8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308 K. (a) If the engine receives 5113 J of heat from the reservoir at T1 in each cycle, how many joules per cycle does it deliver to the reservoir at T2? (b) How much mechanical work is performed by the engine during each cycle? (c) What is the thermal efficiency of the engine?
(5 pts) 15.A quantity of 2.0 moles of an ideal gas undergoes a reversible isothermal process...
 (5 pts) 15.A quantity of 2.0 moles of an ideal gas undergoes a reversible isothermal process (AT -0) at 120 K. In the process 80.0 J of heat energy flows out of the gas. In this process the entropy of the gas a decreases (b) stays the same (c) increases (5 pts) 16. In each cycle a heat engine receives 80.0 J of heat energy from the high temperature reservoir and rejects 30.0 J of heat energy into the low...
(5 pts) 15.A quantity of 2.0 moles of an ideal gas undergoes a reversible isothermal process (AT -0) at 120 K. In the process 80.0 J of heat energy flows out of the gas. In this process the entropy of the gas a decreases (b) stays the same (c) increases (5 pts) 16. In each cycle a heat engine receives 80.0 J of heat energy from the high temperature reservoir and rejects 30.0 J of heat energy into the low...
Problem 1: Two reversible refrigeration cycles are arranged in series. The first cycle receives energy by...
 Problem 1: Two reversible refrigeration cycles are arranged in series. The first cycle receives energy by heat transfer from a cold reservoir at temperature Tc and rejects energy by heat transfer to a reservoir at an intermediate temperature T greater than Te. The second cycle receives energy by heat transfer from the reservoir at temperature T and rejects energy by heat transfer to a higher-temperature reservoir at TH. Obtain an expression for the coefficient of performance of a single reversible...
Problem 1: Two reversible refrigeration cycles are arranged in series. The first cycle receives energy by heat transfer from a cold reservoir at temperature Tc and rejects energy by heat transfer to a reservoir at an intermediate temperature T greater than Te. The second cycle receives energy by heat transfer from the reservoir at temperature T and rejects energy by heat transfer to a higher-temperature reservoir at TH. Obtain an expression for the coefficient of performance of a single reversible...
Operating in series are two reversible heat pumps. Heat transfer gives energy to the first cycle...
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
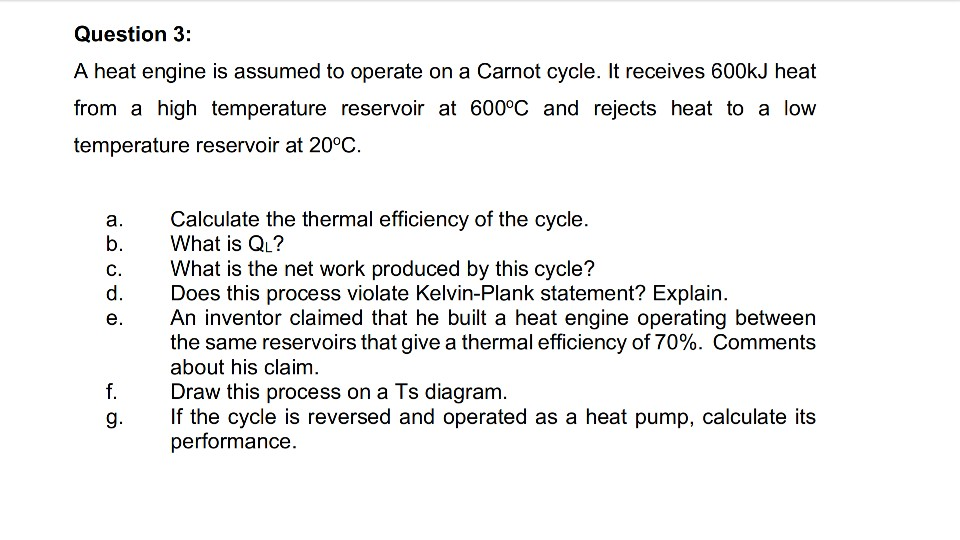 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain...
 Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
A friend passionate about cars mentions that Toyota has developed an engine with a thermal efficiency...
A friend passionate about cars mentions that Toyota has developed an engine with a thermal efficiency of 40% which is an extraordinary accomplishment! 1) Supposing this engine is ideal and based on the Carnot cycle, and also assuming that the cold reservoir temperature is 300 K, what is the hot reservoir temperature to meet the stated efficiency? You mention your result to this friend who points out correctly that the temperature of the hot reservoir would essentially be the temperature...
Most questions answered within 3 hours.
-
Sampling for Engineers Homework question 11.51
Suppose that Y1;Y2;Y3;Y4 denote a random sample from a
Poisson(θ)...
asked 1 minute from now -
Random characters You decide to create a program characters.py
that fills a two-dimensional list with random...
asked 9 minutes ago -
What are the major sources of error in your determination of the
molar mass? Select all...
asked 13 minutes ago -
A. Suppose your manager indicates that for a normally
distributed data set you are analyzing, your...
asked 35 minutes ago -
During the month of August, the average temperature of a lake
next to a local college...
asked 16 minutes ago -
Which of these four researchers is most likely to have made Type
1 error
Kim who...
asked 30 minutes ago -
In a performance test, each of two cars takes 8.9 s to
accelerate from rest to...
asked 31 minutes ago -
Sapphire Aerospace operates 52 weeks per year, and its cost of
goods sold last year was...
asked 34 minutes ago -
Create ER Model
Assets are resources that are used in CMS to display content.
Each asset...
asked 29 minutes ago -
For each the following molecules draw the best possible
structure based on formal charges for each...
asked 35 minutes ago -
A proton moves in the plane of this paper toward the top of the
page. A...
asked 44 minutes ago -
different divisions differing lines of business use
different costs of capital because their cost of equity...
asked 41 minutes ago


 8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
8. A reversible engine, operating in a cycle, withdraws heat from a high temperature (T2=500 K) and heat capacity (C2=20 + 0.001ⓇT [J/mole.k]) reservoir, performs work w, and rejects heat into a low-temperature (T1=300 K) and heat capacity (C=10+ 0.001XT [J/mole.k]) reservoir. Calculate the final temperature of the system and the maximum amount of work.
 (5 pts) 15.A quantity of 2.0 moles of an ideal gas undergoes a reversible isothermal process (AT -0) at 120 K. In the process 80.0 J of heat energy flows out of the gas. In this process the entropy of the gas a decreases (b) stays the same (c) increases (5 pts) 16. In each cycle a heat engine receives 80.0 J of heat energy from the high temperature reservoir and rejects 30.0 J of heat energy into the low...
(5 pts) 15.A quantity of 2.0 moles of an ideal gas undergoes a reversible isothermal process (AT -0) at 120 K. In the process 80.0 J of heat energy flows out of the gas. In this process the entropy of the gas a decreases (b) stays the same (c) increases (5 pts) 16. In each cycle a heat engine receives 80.0 J of heat energy from the high temperature reservoir and rejects 30.0 J of heat energy into the low...
 Problem 1: Two reversible refrigeration cycles are arranged in series. The first cycle receives energy by heat transfer from a cold reservoir at temperature Tc and rejects energy by heat transfer to a reservoir at an intermediate temperature T greater than Te. The second cycle receives energy by heat transfer from the reservoir at temperature T and rejects energy by heat transfer to a higher-temperature reservoir at TH. Obtain an expression for the coefficient of performance of a single reversible...
Problem 1: Two reversible refrigeration cycles are arranged in series. The first cycle receives energy by heat transfer from a cold reservoir at temperature Tc and rejects energy by heat transfer to a reservoir at an intermediate temperature T greater than Te. The second cycle receives energy by heat transfer from the reservoir at temperature T and rejects energy by heat transfer to a higher-temperature reservoir at TH. Obtain an expression for the coefficient of performance of a single reversible...
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
 Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...