Homework Answers



Add Answer to:
B.1 This question concerns the use of different working substances in an engine 2018 (a) Carbon...
6) In a reversible Carnot heat engine which operates between reservoirs at Thigh 500 K and...
 6) In a reversible Carnot heat engine which operates between reservoirs at Thigh 500 K and Tlow = 300 K with a net power output of 600 W, methane gas is used as working fluid. Methane can be considered here as an ideal gas with M 16.043 g mol-, and average heat capacities c 4 R and cp- 5 R. The cycle operates in a steady state with a substance flow rate of 0.375 mol s . Before the isothermal...
6) In a reversible Carnot heat engine which operates between reservoirs at Thigh 500 K and Tlow = 300 K with a net power output of 600 W, methane gas is used as working fluid. Methane can be considered here as an ideal gas with M 16.043 g mol-, and average heat capacities c 4 R and cp- 5 R. The cycle operates in a steady state with a substance flow rate of 0.375 mol s . Before the isothermal...
A Carnot engine operates us ing 1.0 mol e of monoatomic ideal gas as a working...
A Carnot engine operates us ing 1.0 mol e of monoatomic ideal gas as a working s ubstance. In t he first step, the gas is place d in thermal contact with a heat reservoir and expands isothermally to 3 .0 times its initial volume. (a) If the internal energy o f the gas after this step is 6.25 k J , calculate the temperature of the heat reservoir ( T h ) . (b) C alculate the heat absorbed...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
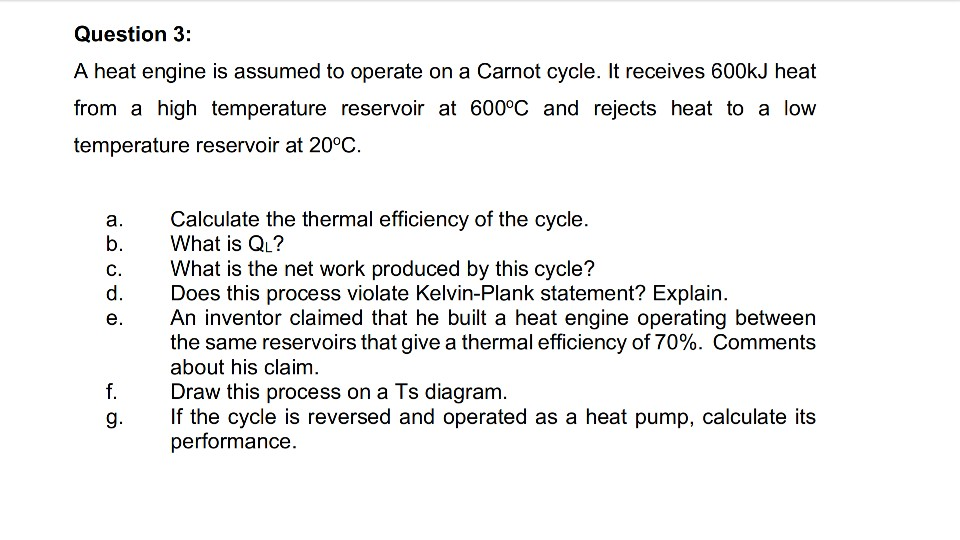 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
During each cycle, a heat engine operating between two heat reservoirs absorbs 156 J from the...
During each cycle, a heat engine operating between two heat reservoirs absorbs 156 J from the reservoir at 100°C and releases 136 J to the reservoir at 20°C. (a) What is the efficiency of this engine? % (b) What is the ratio of its efficiency to that of a Carnot engine working between the same reservoirs? This ratio is called the second law efficiency. εengine / εCarnot =
1) An ideal heat engine employing a carnot cycle Operates between two injerite heat reservoirs with...
 1) An ideal heat engine employing a carnot cycle Operates between two injerite heat reservoirs with the fixed temperatures To 7 To a) The heat engine has an efficiency n = 30% a work generator Find the efficiency of the machine y it is used as a refridgerator b) Braw the diagram of this cycle is T-S co-ords when used as
1) An ideal heat engine employing a carnot cycle Operates between two injerite heat reservoirs with the fixed temperatures To 7 To a) The heat engine has an efficiency n = 30% a work generator Find the efficiency of the machine y it is used as a refridgerator b) Braw the diagram of this cycle is T-S co-ords when used as
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to...
 7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
A heat engine uses 1kg of water as the working fluid.It absorbs heat at 300 C....
 A heat engine uses 1kg of water
as the working fluid.It absorbs heat at 300 C. During the phase the
water goes from saturated liquid to saturated vapor. The heat
rejection phase takes place at 100 C.
Assume a Carnot cycle and do A through E.
Thanks!
A HEAT ENGINE uses 1kg OF WATER AS The wORKING FLUID. IT ABSORBS HEAT AT 300°C. DURWG This Phase Me WATEN Goes FROM SATURATED LIQUID TO SATURA PO VAPOR. The Heat Re Tecnon...
A heat engine uses 1kg of water
as the working fluid.It absorbs heat at 300 C. During the phase the
water goes from saturated liquid to saturated vapor. The heat
rejection phase takes place at 100 C.
Assume a Carnot cycle and do A through E.
Thanks!
A HEAT ENGINE uses 1kg OF WATER AS The wORKING FLUID. IT ABSORBS HEAT AT 300°C. DURWG This Phase Me WATEN Goes FROM SATURATED LIQUID TO SATURA PO VAPOR. The Heat Re Tecnon...
1. (15 points) Consider a Stirling Engine with the following parameters Working gas Isothermal co...
 1. (15 points) Consider a Stirling Engine with the following parameters Working gas Isothermal compression temperature T Isothermal expansion temperature TE Vi Air 80°C 400 °C 650 cm 550 cm3 1000 kPa P1 Operating frequenc Using properties of air at 500K, determine (a) the mass of working gas used in the cycle, (b) the net work done per cycle (in kJ), (c) the net power output produced (in kW), (d) the external heat delivered to the expansion space QE during...
1. (15 points) Consider a Stirling Engine with the following parameters Working gas Isothermal compression temperature T Isothermal expansion temperature TE Vi Air 80°C 400 °C 650 cm 550 cm3 1000 kPa P1 Operating frequenc Using properties of air at 500K, determine (a) the mass of working gas used in the cycle, (b) the net work done per cycle (in kJ), (c) the net power output produced (in kW), (d) the external heat delivered to the expansion space QE during...
102) 2.37 moles of an ideal monatomic gas initially at 255 K undergoes this cycle: It...
102) 2.37 moles of an ideal monatomic gas initially at 255 K undergoes this cycle: It is (1) heated at constant pressure to 655 K, (2) then allowed to cool at constant volume until its temperature returns to its initial value, (3) then compressed isothermally to its initial state. Find: a. the net energy transferred as heat to the gas (excluding the energy transferred as heat out of the gas). b. the net work done by the gas for the...
3. (20 pts) In the Carnot engine (refer to the figure in question 2), an ideal...
3. (20 pts) In the Carnot engine (refer to the figure in question 2), an ideal gas undergoes a cycle of isothermal expansion (A → B), adiabatic expansion (B → C), isothermal compression (C → D), and adiabatic compression (D → A). All processes are assumed to be reversible. The volumes at the points are given that 2VA=VB and VC=2VD. Th is 650 °C and Tc is 30 °C. (1) Calculate the amount of heat added to one mole gas...
Most questions answered within 3 hours.
-
Suppose that on a temperature scale X, water boils at 203.0°X
and freezes at -105.7°X. What...
asked 40 minutes ago -
BaS crystallizes in a cubic unit cell with S2- ions on each
corner and each face....
asked 1 hour ago -
A. 0≤P(Oi)≤10≤P(Oi)≤1 for each i
B. P(Oi)≤0P(Oi)≤0
C. P(Oi)=1+P(OCi)P(Oi)=1+P(OiC)
D. P(Oi)≥1P(Oi)≥1
If an experiment consists of...
asked 2 hours ago -
A battery has an emf of 9.20V and an internal resistance of 1.20
ohm. a)What resistance...
asked 2 hours ago -
The area of an elastic circular loop decreases at a constant
rate, dA/dt = −6.60×10−3 m2/s...
asked 3 hours ago -
The denaturation of proteins can be described by the
equilibrium
F⇌U
where F and U represent...
asked 5 hours ago -
Please answer what the maximum and minimum force is, and the
angle on the ion is...
asked 5 hours ago -
implement a program that reads a number of rows and a symbol.
The program will draw...
asked 5 hours ago -
Assume that when adults with smartphones are randomly selected,
45% use them in meetings or classes....
asked 5 hours ago -
Determine the number of formula units of
Na2SO4 and moles of oxygen contained in 8.11
moles...
asked 5 hours ago -
Explain in steps on the following code
What would be the output when executed
using System;...
asked 5 hours ago -
Given the information in the table, which of the following
statements is CORRECT?
Stock A
Stock...
asked 5 hours ago

 6) In a reversible Carnot heat engine which operates between reservoirs at Thigh 500 K and Tlow = 300 K with a net power output of 600 W, methane gas is used as working fluid. Methane can be considered here as an ideal gas with M 16.043 g mol-, and average heat capacities c 4 R and cp- 5 R. The cycle operates in a steady state with a substance flow rate of 0.375 mol s . Before the isothermal...
6) In a reversible Carnot heat engine which operates between reservoirs at Thigh 500 K and Tlow = 300 K with a net power output of 600 W, methane gas is used as working fluid. Methane can be considered here as an ideal gas with M 16.043 g mol-, and average heat capacities c 4 R and cp- 5 R. The cycle operates in a steady state with a substance flow rate of 0.375 mol s . Before the isothermal...
 1) An ideal heat engine employing a carnot cycle Operates between two injerite heat reservoirs with the fixed temperatures To 7 To a) The heat engine has an efficiency n = 30% a work generator Find the efficiency of the machine y it is used as a refridgerator b) Braw the diagram of this cycle is T-S co-ords when used as
1) An ideal heat engine employing a carnot cycle Operates between two injerite heat reservoirs with the fixed temperatures To 7 To a) The heat engine has an efficiency n = 30% a work generator Find the efficiency of the machine y it is used as a refridgerator b) Braw the diagram of this cycle is T-S co-ords when used as
 7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
 A heat engine uses 1kg of water
as the working fluid.It absorbs heat at 300 C. During the phase the
water goes from saturated liquid to saturated vapor. The heat
rejection phase takes place at 100 C.
Assume a Carnot cycle and do A through E.
Thanks!
A HEAT ENGINE uses 1kg OF WATER AS The wORKING FLUID. IT ABSORBS HEAT AT 300°C. DURWG This Phase Me WATEN Goes FROM SATURATED LIQUID TO SATURA PO VAPOR. The Heat Re Tecnon...
A heat engine uses 1kg of water
as the working fluid.It absorbs heat at 300 C. During the phase the
water goes from saturated liquid to saturated vapor. The heat
rejection phase takes place at 100 C.
Assume a Carnot cycle and do A through E.
Thanks!
A HEAT ENGINE uses 1kg OF WATER AS The wORKING FLUID. IT ABSORBS HEAT AT 300°C. DURWG This Phase Me WATEN Goes FROM SATURATED LIQUID TO SATURA PO VAPOR. The Heat Re Tecnon...
 1. (15 points) Consider a Stirling Engine with the following parameters Working gas Isothermal compression temperature T Isothermal expansion temperature TE Vi Air 80°C 400 °C 650 cm 550 cm3 1000 kPa P1 Operating frequenc Using properties of air at 500K, determine (a) the mass of working gas used in the cycle, (b) the net work done per cycle (in kJ), (c) the net power output produced (in kW), (d) the external heat delivered to the expansion space QE during...
1. (15 points) Consider a Stirling Engine with the following parameters Working gas Isothermal compression temperature T Isothermal expansion temperature TE Vi Air 80°C 400 °C 650 cm 550 cm3 1000 kPa P1 Operating frequenc Using properties of air at 500K, determine (a) the mass of working gas used in the cycle, (b) the net work done per cycle (in kJ), (c) the net power output produced (in kW), (d) the external heat delivered to the expansion space QE during...