Homework Answers
Add Answer to:
[3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir...
A Carnot engine has an efficiency of 0.445, and the temperature of its cold reservoir is...
A Carnot engine has an efficiency of 0.445, and the temperature of its cold reservoir is 434 K. (a) Determine the temperature of its hot reservoir. (b) If 5610 J of heat is rejected to the cold reservoir, what amount of heat is put into the engine?
A Carnot engine operates with an efficiency of 29% when the temperature of its cold reservoir...
A Carnot engine operates with an efficiency of 29% when the temperature of its cold reservoir is 295 K. Assuming that the temperature of the hot reservoir remains the same, what must be the temperature of the cold reservoir in order to increase the efficiency to 33%?
1. A Carnot engine has a hot reservoir at 250 degrees C, and a a cold...
1. A Carnot engine has a hot reservoir at 250 degrees C, and a a cold reservoir at 25.0 degrees C. What is the efficiency of this engine? A) 10% B) 43% C) 57% D) 90% 2. A heat engine does 50 J of work and dumps 30 J of heat into the cold reservoir. How much heat was absorbed from the hot reservoir? A) 20 J B) 30 J C) 50 J D) 80 J 3. A heat engine...
1.The efficiency of a Carnot engine is 27%. The engine absorbs 826 J of energy per...
1.The efficiency of a Carnot engine is 27%. The engine absorbs 826 J of energy per cycle by heat from a hot reservoir at 503 K. (a) Determine the energy expelled per cycle. _ J (b) Determine the temperature of the cold reservoir. _K 2.A sample of helium behaves as an ideal gas as energy is added by heat at constant pressure from 273 K to 343 K. If 15.0 J of work is done by the gas during this...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
+ -/24 points Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat...
 + -/24 points Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat...
+ -/24 points Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
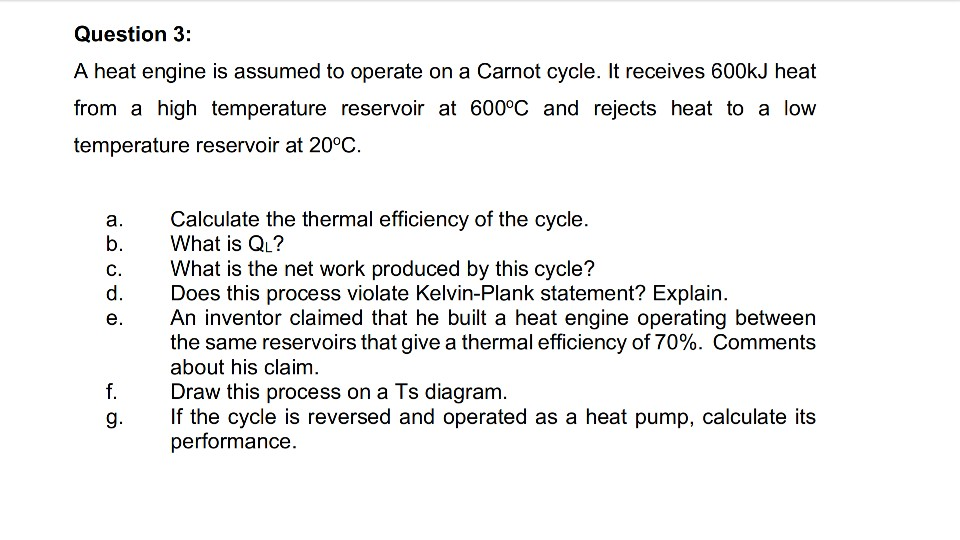 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
second law of thermodynamics
A refrigerator is coupled with a Carnot heat engine as a system. The heat engine absorbs heatfrom a hot reservoir at 220°C and rejects waste heat to cold reservoir at 25°C. The absorbedheat is converted to work by the heat engine and supply to the refrigerator to produced ice.The ice is formed at cold reservoir of refrigerator at 0°C and the refrigerator rejected 63 kW ofheat to the hot reservoir at 25°C. By assuming the system operates based on the...
Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a...
 Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use...
Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use...
Suppose a heat engine has an efficiency of 0.177 and produces 5.40×103 J of work. How...
Suppose a heat engine has an efficiency of 0.177 and produces 5.40×103 J of work. How much energy is pulled in from the hot reservoir? How much energy is rejected by the engine? (This energy is "exhausted" to the cold reservoir.))
Most questions answered within 3 hours.
-
Calculate the number density of argon gas at a temperature of
24C and a pressure of...
asked 1 hour ago -
Alternative
Classification
How to Estimate
Probabilities from Data? ( For continuous Attributes)
And How to generate...
asked 1 hour ago -
An explosion breaks a 20.0-kg object into three parts. The
object is initially moving at a...
asked 2 hours ago -
Calculate the approximate number of residues of Rubisco, which
is involved in carbon fixation in plants,...
asked 3 hours ago -
Other decisions about scientific claims can have a much broader
impact.ENERGYarrow-10x10.png, environment, health, security - all...
asked 4 hours ago -
I need to write a research paper and work cited about this
topic: The United States...
asked 4 hours ago -
Hello! I was wondering if I could have some help?
If the vapor pressure of carvone...
asked 5 hours ago -
An economist wants to estimate the mean per capita income (in
thousands of dollars) for a...
asked 5 hours ago -
What would be the input/output characteristic of a circuit
obtained by putting two of your 2's-complementers...
asked 5 hours ago -
In Drosophila, the transition from the syncytial blastoderm
stage to the cellular blastoderm stage is a...
asked 5 hours ago -
Project management question:
Name 3 different types of resources (hint: humans are one
type)
asked 5 hours ago -
Consider the following reaction: C 2H 2( g) + 2H 2( g) C 2H 6(
g)...
asked 6 hours ago
![[3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir is at 0° C. (a) Assuming this engine follows the Carnot cycle, what is the temperature of the hot reservoir? (b) Suppose the heat input to this engine was 10 J. Calculate the work done by this engine c) Suppose the heat input to this engine was 5). Calculate the heat rejected by this engine.](http://img.homeworklib.com/questions/2bb1b0f0-8da2-11eb-90b7-7f0fc38fd200.png?x-oss-process=image/resize,w_560)

 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 + -/24 points Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat...
+ -/24 points Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat...
 Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use...
Complete Analysis of Heat Engine Goal Solve for the efficiency of a heat engine using a five-step process the includes: 1. Making a state table. 2. Making a process table. 3. Calculating the totals for Work, Heat, and Internal-Energy-Change. 4. Identifying the heat input (hot reservoir) and output (cold reservoir). 5. Calculating the efficiency of the engine. isothermal Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use...