A Carnot engine extracts 910J from a 550K reservoir during each cycle and rejects 500J to...
A Carnot engine extracts 910J from a 550K reservoir during each cycle and rejects 500J to a cooler reservoir. It operates at 22 cycles per second.
Find the work done during each cycle.
Find its efficiency.
Find the temperature of the cool reservoir.
Find its mechanical power output.
Homework Answers
a)910 - 500 = 410 J
b) e = 1 - (Qh/Qc) = 1 - (500/910) = 0.4505
c)500/910 = Tc/Th = Tc/550 ==> Tc = 302.2 K
d)P = W/T = 410/(1/22) = 9020 W
Add Answer to:
A Carnot engine extracts 910J from a 550K reservoir during each
cycle and rejects 500J to...
1) 150 g of a liquid at 45°C is filled in an insulated metal container at...
 1) 150 g of a liquid at 45°C is filled in an insulated metal container at 35°C whose mass is 110 g. The system eventually reaches an equilibrium. Find: (a) the final equilibrium temperature, and (b) estimate total change in entropy of the system (i.e. metal container plus liquid). Specific heat of liquid is 4186 J/kg.Cº and specific heat of metal is 900 J/kg.C. 2) A Carnot engine working between a hot and cool reservoir, extracts 800 J from a...
1) 150 g of a liquid at 45°C is filled in an insulated metal container at 35°C whose mass is 110 g. The system eventually reaches an equilibrium. Find: (a) the final equilibrium temperature, and (b) estimate total change in entropy of the system (i.e. metal container plus liquid). Specific heat of liquid is 4186 J/kg.Cº and specific heat of metal is 900 J/kg.C. 2) A Carnot engine working between a hot and cool reservoir, extracts 800 J from a...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
A Carnot heat engine operates between reservoirs at 164 ∘C and 0∘C. If the engine extracts...
A Carnot heat engine operates between reservoirs at 164 ∘C and 0∘C. If the engine extracts 22 J of energy from the hot reservoir per cycle, how many cycles will it take to lift a 12 kg mass a height of 10 m?
3. An ideal Carnot engine has an input of 150 J of heat per cycle at...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
Ans. During one aycle, an engine extracts 2.00 X 10' J of energy from a hot...
 Ans. During one aycle, an engine extracts 2.00 X 10' J of energy from a hot reservoir transfers 1.5 X 103 J to a cold reservoir. 2. and a. Find the thermal efficiency of the engine Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 see?
Ans. During one aycle, an engine extracts 2.00 X 10' J of energy from a hot reservoir transfers 1.5 X 103 J to a cold reservoir. 2. and a. Find the thermal efficiency of the engine Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 see?
Ans. 2. During one cyele, an engine extracts 2.00 x 10' J of energy from a...
 Ans. 2. During one cyele, an engine extracts 2.00 x 10' J of energy from a hot reservoir and transfers 1.5 X 103 J to a cold reservoir. a. Find the thermal efficiency of the engine. Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 sec? Ans.
Ans. 2. During one cyele, an engine extracts 2.00 x 10' J of energy from a hot reservoir and transfers 1.5 X 103 J to a cold reservoir. a. Find the thermal efficiency of the engine. Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 sec? Ans.
A heat engine operates in a Carnot cycle between 86.0°C and 345°C. It absorbs 20,000 J...
A heat engine operates in a Carnot cycle between 86.0°C and 345°C. It absorbs 20,000 J of energy per cycle from the hot reservoir. The duration of each cycle is 5.00 s. (a) What is the mechanical power output of this engine? (b) How much energy does it expel in each cycle by heat?
An ideal refrigerator extracts 410 joules of heat from a reservoir at 290 K and rejects...
An ideal refrigerator extracts 410 joules of heat from a reservoir at 290 K and rejects heat to a reservoir at 488 K. What is the ideal coefficient of performance and how much work is done in each cycle?
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
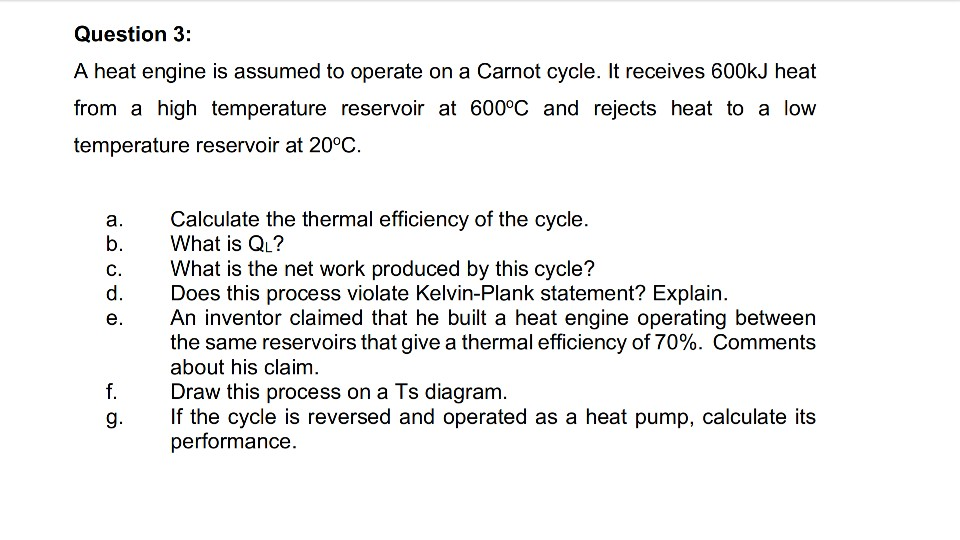 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
An ideal refrigerator extracts 420 joules of heat from a reservoir at 280 K and rejects...
 An ideal refrigerator extracts 420 joules of heat from a reservoir at 280 K and rejects heat to a reservoir at 498 K. What is the ideal coefficient of performance and how much work is done in each cycle? COP = Number Work = Number Joules
An ideal refrigerator extracts 420 joules of heat from a reservoir at 280 K and rejects heat to a reservoir at 498 K. What is the ideal coefficient of performance and how much work is done in each cycle? COP = Number Work = Number Joules
Most questions answered within 3 hours.
-
Given P(Ec ) = 0.43, P(F) = 0.52, and P(EF) = 0.18.
Find P( E |...
asked 26 minutes ago -
Consider two empty containers A and B whose volumes are
10mL and 20mL respectively. 1mL of...
asked 30 minutes ago -
QUESTION 6
Determine the linear momentum of a 2,800 kg houseboat going 3
m/s.
9,100 kg.m/s...
asked 44 minutes ago -
Jor-el throws a ball upward from the top of a 728 foot building
on the planet...
asked 48 minutes ago -
Which of the following will most likely to happen if Federal
Reserve Bank decreases the money...
asked 30 minutes ago -
You’ve just joined the investment banking firm of Dewey,
Cheatum, and Howe. They’ve offered you two...
asked 24 minutes ago -
An air conditioner cools 226 m^3/min of humid air at 36 oC and
98% relative humidity...
asked 24 minutes ago -
Vaughn Manufacturing acquires a coal mine at a cost of $1870000.
Intangible development costs total $354000....
asked 33 minutes ago -
Question 5
What effect would a decrease in
temperature have on pressure, assuming that volume
(T)...
asked 46 minutes ago -
Draw the Lewis dot structures for the following molecules. None
of the atoms have a formal...
asked 50 minutes ago -
What does it mean when an element is radioactive?
a.
It means the element is changing...
asked 50 minutes ago -
A company deposits $6,000 in a bank at the end of every year for
10 years....
asked 50 minutes ago
 1) 150 g of a liquid at 45°C is filled in an insulated metal container at 35°C whose mass is 110 g. The system eventually reaches an equilibrium. Find: (a) the final equilibrium temperature, and (b) estimate total change in entropy of the system (i.e. metal container plus liquid). Specific heat of liquid is 4186 J/kg.Cº and specific heat of metal is 900 J/kg.C. 2) A Carnot engine working between a hot and cool reservoir, extracts 800 J from a...
1) 150 g of a liquid at 45°C is filled in an insulated metal container at 35°C whose mass is 110 g. The system eventually reaches an equilibrium. Find: (a) the final equilibrium temperature, and (b) estimate total change in entropy of the system (i.e. metal container plus liquid). Specific heat of liquid is 4186 J/kg.Cº and specific heat of metal is 900 J/kg.C. 2) A Carnot engine working between a hot and cool reservoir, extracts 800 J from a...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
 Ans. During one aycle, an engine extracts 2.00 X 10' J of energy from a hot reservoir transfers 1.5 X 103 J to a cold reservoir. 2. and a. Find the thermal efficiency of the engine Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 see?
Ans. During one aycle, an engine extracts 2.00 X 10' J of energy from a hot reservoir transfers 1.5 X 103 J to a cold reservoir. 2. and a. Find the thermal efficiency of the engine Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 see?
 Ans. 2. During one cyele, an engine extracts 2.00 x 10' J of energy from a hot reservoir and transfers 1.5 X 103 J to a cold reservoir. a. Find the thermal efficiency of the engine. Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 sec? Ans.
Ans. 2. During one cyele, an engine extracts 2.00 x 10' J of energy from a hot reservoir and transfers 1.5 X 103 J to a cold reservoir. a. Find the thermal efficiency of the engine. Ans. b. How much work does the engine do in one cycle? Ans. c. How much power does the engine generate if it goes through four cycles in 2.5 sec? Ans.
 An ideal refrigerator extracts 420 joules of heat from a reservoir at 280 K and rejects heat to a reservoir at 498 K. What is the ideal coefficient of performance and how much work is done in each cycle? COP = Number Work = Number Joules
An ideal refrigerator extracts 420 joules of heat from a reservoir at 280 K and rejects heat to a reservoir at 498 K. What is the ideal coefficient of performance and how much work is done in each cycle? COP = Number Work = Number Joules