please answer these questions 1. What is the difference between a refrigerator and a heat pump?...
please answer these questions
1. What is the difference between a refrigerator and a
heat pump?
2. A heat pump and a refrigerator are operating between the same
two thermal
reservoirs. Which one has a higher COP?
3. Define the COP of a heat pump.
4. Thermal efficiency of a heat engine is defined to be the ratio
of energy output
desirable to energy input. Would you think that efficiency is an
appropriate definition
to be used when a heat pump is being discussed? Why?
5. Is the COP of a heat pump always larger than 1?
6. Indicate whether the following statements are true or
false:
7. A process that causes heat to be removed from one reservoir only
is feasible.
8. A process that causes heat to be supplied to one reservoir only
is feasible.
9. A process that causes heat to be transferred from reservoir A to
reservoir B is feasible.
10. For a heat pump, COP<1.
11. For a refrigerator, COP<1
Homework Answers



Add Answer to:
please answer these questions
1. What is the difference between a refrigerator and a
heat pump?...
QUESTION 1 A heat pump extracts heat from a cold temperature reservoir at 263 K at...
 QUESTION 1 A heat pump extracts heat from a cold temperature reservoir at 263 K at high temperature reservoir, an auditorium, at 298 K. The heat pump requires (a) Draw a schematic diagram of the heat pump a rate of Q, and rejects 8 kW of heat to a an input power of 2.5 kw system and the reservoirs and show all energies and their values and directions on the diagram. ing an energy balance on the system, determine the...
QUESTION 1 A heat pump extracts heat from a cold temperature reservoir at 263 K at high temperature reservoir, an auditorium, at 298 K. The heat pump requires (a) Draw a schematic diagram of the heat pump a rate of Q, and rejects 8 kW of heat to a an input power of 2.5 kw system and the reservoirs and show all energies and their values and directions on the diagram. ing an energy balance on the system, determine the...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain...
 Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
A Carnot engine works between two heat reservoirs at temperatures Thot=300.0K and Tcold=230.0K. What is its...
A Carnot engine works between two heat reservoirs at temperatures Thot=300.0K and Tcold=230.0K. What is its efficiency? If it absorbs 140.0J from the hot reservoir during each cycle, how much work does it do? How much heat does it reject during each cycle? What is the coefficient of perfomance, COP, of this engine when it works as a refrigerator between the same two reservoirs?
A Carnot engine works between two heat reservoirs at temperatures Th = 360 K and Tc...
A Carnot engine works between two heat reservoirs at temperatures Th = 360 K and Tc = 240 K. (a) What is its efficiency? % (b) If it absorbs 95 J from the hot reservoir during each cycle, how much work does it do? J (c) How much heat does it give off during each cycle? J (d) What is the COP of this engine when it works as a refrigerator between the same two reservoirs?
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write...
 8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
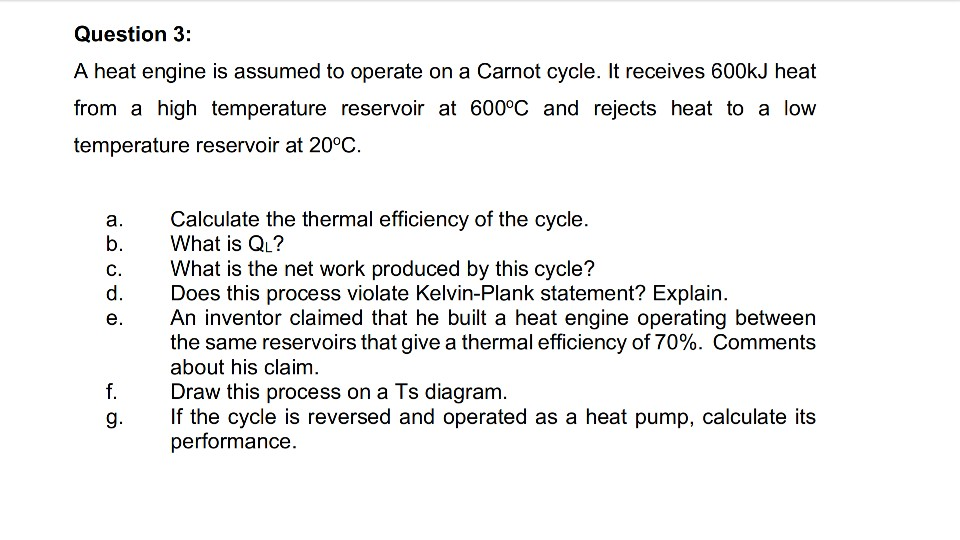 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
Please help 17. What is AU for a cyclical process? 18. For a refrigerator, where is...
 Please help
17. What is AU for a cyclical process? 18. For a refrigerator, where is the hot reservoir and where is the cold reservoir? 19. For a heat pump, where is the hot reservoir and where is the cold reservoir? 20. An inventor claims that they have created an engine that is more efficient than a Carnot engi between the same two temperatures. Why are they lying?
Please help
17. What is AU for a cyclical process? 18. For a refrigerator, where is the hot reservoir and where is the cold reservoir? 19. For a heat pump, where is the hot reservoir and where is the cold reservoir? 20. An inventor claims that they have created an engine that is more efficient than a Carnot engi between the same two temperatures. Why are they lying?
7. We decide to store 200 MJ of electrical energy by driving a heat pump which...
 7. We decide to store 200 MJ of electrical energy by driving a heat pump which transfers heat from 20°C to molten salt at 500°C. This energy is retrieved later by running a heat engine between the same two temperatures. Both the heat pump and the engine can be described by efficiencies WIQH, and they both have the same ideal (Carnot) efficiency. A. What is the ideal efficiency of the heat pump? B. molten salt? Assuming ideal efficiency, how much...
7. We decide to store 200 MJ of electrical energy by driving a heat pump which transfers heat from 20°C to molten salt at 500°C. This energy is retrieved later by running a heat engine between the same two temperatures. Both the heat pump and the engine can be described by efficiencies WIQH, and they both have the same ideal (Carnot) efficiency. A. What is the ideal efficiency of the heat pump? B. molten salt? Assuming ideal efficiency, how much...
Most questions answered within 3 hours.
-
Industry: Telecommunications in India
Here, you need to discuss the sources of the growth pattern
and...
asked 1 hour ago -
Hydrobromic acid
dissolves solid iron according to the followiaung reaction:
Fe(s)+2HBr(aq)→
FeBr2(aq)+ H2(g)
Part A. What...
asked 2 hours ago -
Verify the following vector identity in SPHERICAL
COORDINATES.
div(curl(A)) = 0
asked 4 hours ago -
The work function (Φ) for a metal is 7.50×10-19 J.
What is the longest wavelength (nm)...
asked 5 hours ago -
1. Would the following procedural changes cause the
calculated mass percent Ni2+ ion in Ni(NH3)nCl2 to...
asked 6 hours ago -
1. Why does the voltage of the capacitor vary with frequency?
where does this missing voltage...
asked 8 hours ago -
p p please write each step out clearly
3. A manager of a company is considering...
asked 6 hours ago -
On October 1, Ebony Ernst organized Ernst Consulting; on October
3, the owner contributed $83,540 in...
asked 8 hours ago -
A.)As an electron moves through a region of space, its speed
decreases from 9.60 × 106...
asked 8 hours ago -
In java with the netbeans program do:
Through object programming
Create a class "ContadorP", which is...
asked 8 hours ago -
Pattern Corporation acquired all of Science
Company's outstanding stock on January 1, 2016 for $600,000 cash....
asked 8 hours ago -
Here are all accounts Tyler Co. maintains.
Tax expenses $4,750
Common stocks $48,840
SG&A expenses $2,500...
asked 8 hours ago
 QUESTION 1 A heat pump extracts heat from a cold temperature reservoir at 263 K at high temperature reservoir, an auditorium, at 298 K. The heat pump requires (a) Draw a schematic diagram of the heat pump a rate of Q, and rejects 8 kW of heat to a an input power of 2.5 kw system and the reservoirs and show all energies and their values and directions on the diagram. ing an energy balance on the system, determine the...
QUESTION 1 A heat pump extracts heat from a cold temperature reservoir at 263 K at high temperature reservoir, an auditorium, at 298 K. The heat pump requires (a) Draw a schematic diagram of the heat pump a rate of Q, and rejects 8 kW of heat to a an input power of 2.5 kw system and the reservoirs and show all energies and their values and directions on the diagram. ing an energy balance on the system, determine the...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
Question 12 PHYSICS 120 (a) Carefully explain the difference between irreversible and reversible processes. Also explain what the second law of thermodynamics dictates about reversible processes. (You may find it helpful to compare water freezing at 0 °C and super- cooled water freezing at-5 °C.) [5 marks A heat engine operates with an efficiency n = 0.30 between two energy reservoirs at temperatures of 450 K and 293 K. The engine does 90 J of work per cycle. (b) Draw...
 8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
 Please help
17. What is AU for a cyclical process? 18. For a refrigerator, where is the hot reservoir and where is the cold reservoir? 19. For a heat pump, where is the hot reservoir and where is the cold reservoir? 20. An inventor claims that they have created an engine that is more efficient than a Carnot engi between the same two temperatures. Why are they lying?
Please help
17. What is AU for a cyclical process? 18. For a refrigerator, where is the hot reservoir and where is the cold reservoir? 19. For a heat pump, where is the hot reservoir and where is the cold reservoir? 20. An inventor claims that they have created an engine that is more efficient than a Carnot engi between the same two temperatures. Why are they lying?
 7. We decide to store 200 MJ of electrical energy by driving a heat pump which transfers heat from 20°C to molten salt at 500°C. This energy is retrieved later by running a heat engine between the same two temperatures. Both the heat pump and the engine can be described by efficiencies WIQH, and they both have the same ideal (Carnot) efficiency. A. What is the ideal efficiency of the heat pump? B. molten salt? Assuming ideal efficiency, how much...
7. We decide to store 200 MJ of electrical energy by driving a heat pump which transfers heat from 20°C to molten salt at 500°C. This energy is retrieved later by running a heat engine between the same two temperatures. Both the heat pump and the engine can be described by efficiencies WIQH, and they both have the same ideal (Carnot) efficiency. A. What is the ideal efficiency of the heat pump? B. molten salt? Assuming ideal efficiency, how much...