Homework Answers
2.1 Cinnamic Acid:

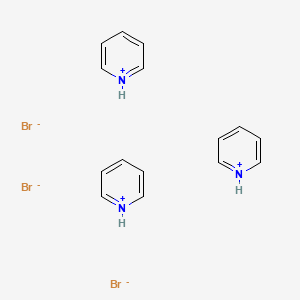
Pyridinium tribromide:
Reaction:
Add Answer to:
2. Cover Page Questions 2.1. Draw the chemical structures of cinnamic acid, pyridinium tribromide and acetic...
0.8002 g of (E)-cinnamic acid, 10 mL of glacial acetic acid and 1.7610g of pyridinium tribromide...
0.8002 g of (E)-cinnamic acid, 10 mL of glacial acetic acid and 1.7610g of pyridinium tribromide was heated under reflux to demonstrate the bromination of (E)-cinnamic acid. The mass of the final product was 3.4143 g and the melting point range was 175.4-203.4 ℃. What is the theoretical yield, actual yield, percent yield? Please show work! (Pyridinium tribromide and cinnamic reaction react in a 1:1 ratio).
Suppose a student started with 133.0 mg of trans-cinnamic acid, 443 mg of pyridinium tribromide, and...
Suppose a student started with 133.0 mg of trans-cinnamic acid, 443 mg of pyridinium tribromide, and 2.60 mL of glacial acetic acid. After the reaction and workup, the student ended up with 0.1683 g of brominated product. Calculate the student\'s theoretical and percent yields.
Pre-Laboratory Assignment 1. What safety precautions must be observed when using (a) pyridinium tribromide? (b) acetic...
 Pre-Laboratory Assignment 1. What safety precautions must be observed when using (a) pyridinium tribromide? (b) acetic acid? 2. Calculate the theoretical yield for the bromination of both stilbenes and cinamic acid, assuming the presence of excess pyridinium tribromide. Note the theoretical yields here and in your laboratory notebook 3. Draw the mechanism, including the intermediate bromonium ion, generated in the bromination of trans-2-butene. 4. (a) Look up and draw structures for cinnamic acid, cis-stilbene, and trans-stilbene. (b) Predict the relative...
Pre-Laboratory Assignment 1. What safety precautions must be observed when using (a) pyridinium tribromide? (b) acetic acid? 2. Calculate the theoretical yield for the bromination of both stilbenes and cinamic acid, assuming the presence of excess pyridinium tribromide. Note the theoretical yields here and in your laboratory notebook 3. Draw the mechanism, including the intermediate bromonium ion, generated in the bromination of trans-2-butene. 4. (a) Look up and draw structures for cinnamic acid, cis-stilbene, and trans-stilbene. (b) Predict the relative...
here is the procedure if needed EXPERIMENT 8: BROMINATION OF TRANS-STILBENE glacial acetic acid Objective: Execute...
 here is the procedure if needed
EXPERIMENT 8: BROMINATION OF TRANS-STILBENE glacial acetic acid Objective: Execute first step of two-step sequence to transform an alkene into an alkyne via a dihalide intermediate and use infrared spectroscopy to identify functional groups PRELAB NOTEBOOK: In the laboratory notebook, write the overall experimental objective, chemical equation(s), reaction mechanism (consider all possible stereoisomers), draw a diagram or outline of the procedural steps, and complete the chemical safety table for all chemicals. 1. Why is...
here is the procedure if needed
EXPERIMENT 8: BROMINATION OF TRANS-STILBENE glacial acetic acid Objective: Execute first step of two-step sequence to transform an alkene into an alkyne via a dihalide intermediate and use infrared spectroscopy to identify functional groups PRELAB NOTEBOOK: In the laboratory notebook, write the overall experimental objective, chemical equation(s), reaction mechanism (consider all possible stereoisomers), draw a diagram or outline of the procedural steps, and complete the chemical safety table for all chemicals. 1. Why is...
1. Using Sigma-Aldrich website or Merck Index website or Google search find and draw structures for...
 1. Using Sigma-Aldrich website or Merck Index website or Google search find and draw structures for the following compounds. (a) Atropine (b) Hydroxychloroquine (c) Saccharin (d) Cholesterol (e) Vitamin C (ascorbic acid) 2. Find the melting points for the following compounds by using Sigma-Aldrich or Google search (a) Biphenyl (b) 4-bromobenzoic acid (C) 3-nitrophenol 9. Chlorine, bromine and iodine all react with alkenes such as ethene in an addition reaction (a) Write the equation for the bromination of ethene, and...
1. Using Sigma-Aldrich website or Merck Index website or Google search find and draw structures for the following compounds. (a) Atropine (b) Hydroxychloroquine (c) Saccharin (d) Cholesterol (e) Vitamin C (ascorbic acid) 2. Find the melting points for the following compounds by using Sigma-Aldrich or Google search (a) Biphenyl (b) 4-bromobenzoic acid (C) 3-nitrophenol 9. Chlorine, bromine and iodine all react with alkenes such as ethene in an addition reaction (a) Write the equation for the bromination of ethene, and...
Most questions answered within 3 hours.
-
Using MARS simulator, write MIPS programs according to
the following scenarios: Receive a positive integer number...
asked 1 hour ago -
An object in front of a concave mirror has a real image that is
11.5 cm...
asked 1 hour ago -
Consider the reaction, C3 H8 + O2 --> CO2 + H2O. How many
moles of O2...
asked 3 hours ago -
You and your opponent both roll a fair die. If you both roll the
same number,...
asked 3 hours ago -
In a study of the accuracy of fast food drive-through orders,
Restaurant A had 257 accurate...
asked 3 hours ago -
Identify and describe in detail the four categories of
institutions that could be included in a...
asked 4 hours ago -
In python
class Customer:
def __init__(self, customer_id, last_name, first_name, phone_number, address):
self._customer_id = int(customer_id)
self._last_name =...
asked 4 hours ago -
What is an example of a limitation in implementing a new
ERP system and how it...
asked 4 hours ago -
In a section of 9.7cm of an artery with a radius of 2.6mm there
is a...
asked 4 hours ago -
the two carboxylic acid groups of aspartic acid have different
acidities with pKa values of 2.1...
asked 4 hours ago -
Would CuCO3 aqueous salt combined with calcium chloride
form a solid precipitate? If so, what would...
asked 4 hours ago -
How do ECM Solutions assist in embedding a culture of continuous
improvement in an organization? (Project...
asked 4 hours ago

 Pre-Laboratory Assignment 1. What safety precautions must be observed when using (a) pyridinium tribromide? (b) acetic acid? 2. Calculate the theoretical yield for the bromination of both stilbenes and cinamic acid, assuming the presence of excess pyridinium tribromide. Note the theoretical yields here and in your laboratory notebook 3. Draw the mechanism, including the intermediate bromonium ion, generated in the bromination of trans-2-butene. 4. (a) Look up and draw structures for cinnamic acid, cis-stilbene, and trans-stilbene. (b) Predict the relative...
Pre-Laboratory Assignment 1. What safety precautions must be observed when using (a) pyridinium tribromide? (b) acetic acid? 2. Calculate the theoretical yield for the bromination of both stilbenes and cinamic acid, assuming the presence of excess pyridinium tribromide. Note the theoretical yields here and in your laboratory notebook 3. Draw the mechanism, including the intermediate bromonium ion, generated in the bromination of trans-2-butene. 4. (a) Look up and draw structures for cinnamic acid, cis-stilbene, and trans-stilbene. (b) Predict the relative...
 here is the procedure if needed
EXPERIMENT 8: BROMINATION OF TRANS-STILBENE glacial acetic acid Objective: Execute first step of two-step sequence to transform an alkene into an alkyne via a dihalide intermediate and use infrared spectroscopy to identify functional groups PRELAB NOTEBOOK: In the laboratory notebook, write the overall experimental objective, chemical equation(s), reaction mechanism (consider all possible stereoisomers), draw a diagram or outline of the procedural steps, and complete the chemical safety table for all chemicals. 1. Why is...
here is the procedure if needed
EXPERIMENT 8: BROMINATION OF TRANS-STILBENE glacial acetic acid Objective: Execute first step of two-step sequence to transform an alkene into an alkyne via a dihalide intermediate and use infrared spectroscopy to identify functional groups PRELAB NOTEBOOK: In the laboratory notebook, write the overall experimental objective, chemical equation(s), reaction mechanism (consider all possible stereoisomers), draw a diagram or outline of the procedural steps, and complete the chemical safety table for all chemicals. 1. Why is...
 1. Using Sigma-Aldrich website or Merck Index website or Google search find and draw structures for the following compounds. (a) Atropine (b) Hydroxychloroquine (c) Saccharin (d) Cholesterol (e) Vitamin C (ascorbic acid) 2. Find the melting points for the following compounds by using Sigma-Aldrich or Google search (a) Biphenyl (b) 4-bromobenzoic acid (C) 3-nitrophenol 9. Chlorine, bromine and iodine all react with alkenes such as ethene in an addition reaction (a) Write the equation for the bromination of ethene, and...
1. Using Sigma-Aldrich website or Merck Index website or Google search find and draw structures for the following compounds. (a) Atropine (b) Hydroxychloroquine (c) Saccharin (d) Cholesterol (e) Vitamin C (ascorbic acid) 2. Find the melting points for the following compounds by using Sigma-Aldrich or Google search (a) Biphenyl (b) 4-bromobenzoic acid (C) 3-nitrophenol 9. Chlorine, bromine and iodine all react with alkenes such as ethene in an addition reaction (a) Write the equation for the bromination of ethene, and...