Does it matter which of the two hybrid orbitals are used to hold the two nonbonding electron pairs?
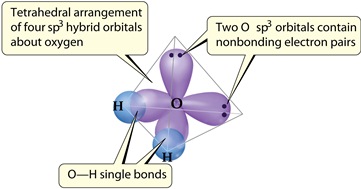
| Yes. Hybrids aren't equivalent. Hybrid formed from px orbital holds the two nonbonding electron pairs. |
| Yes. Hybrids aren't equivalent. Hybrid formed from pz orbital holds the two nonbonding electron pairs. |
| Yes. Hybrids aren't equivalent. Hybrid formed from py orbital holds the two nonbonding electron pairs. |
| No. All four hybrids are equivalent and the angles between them are all the same, so we can use any of the two to hold the nonbonding pairs. |
Homework Answers
Does it matter which of the two sp3 hybrid orbitals are used to hold the two non bonding electron pairs?
No. All four hybrids are equivalent and the angles between them are all the same, so we can use any of the two to hold the non bonding pairs.
Explanation:
Oxygen in the two hybrid orbitals consists of lone pairs, the remaining two overlaps with the 1s orbitals of hydrogen atoms forming the O-H Bonds in H2O.
Add Answer to:
Does it matter which of the two hybrid orbitals are
used to hold the two nonbonding electron...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron...
 Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbital on the central O atom makes up the sigma bond between this O and an outer H atom in water, H,O? orbital on o What is the approximate H-O-H bond angle? Sigma Bonding A o bond arises from...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbital on the central O atom makes up the sigma bond between this O and an outer H atom in water, H,O? orbital on o What is the approximate H-O-H bond angle? Sigma Bonding A o bond arises from...
(e) a- use of s and p atomic orbitals b- use of s, p, and d...
 (e)
a- use of s and p atomic orbitals
b- use of s, p, and d atomic orbitals
c-use of only s orbitals
d-generation of more than four hybrid orbitals
Hybrid Orbitals Use the animation to answer the following questions. (a) Mixing yellow and blue atomic orbitals can produce: 2, 3 or 4 hybrid orbitals. O 4 or 5 hybrid orbitals. 1 or 2 hybrid orbital(s). O 5 or 6 hybrid orbitals. (b) Mixing one yellow atomic orbital and one...
(e)
a- use of s and p atomic orbitals
b- use of s, p, and d atomic orbitals
c-use of only s orbitals
d-generation of more than four hybrid orbitals
Hybrid Orbitals Use the animation to answer the following questions. (a) Mixing yellow and blue atomic orbitals can produce: 2, 3 or 4 hybrid orbitals. O 4 or 5 hybrid orbitals. 1 or 2 hybrid orbital(s). O 5 or 6 hybrid orbitals. (b) Mixing one yellow atomic orbital and one...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron...
 Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density 5 lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbitals make up the sigma bond between Sand F in sulfur hexafluoride, SF6? orbital on S+ orbital on s o orbital on F rbital on F What are the approximate F-S-F bond angles ? (list all possible) 90...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density 5 lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbitals make up the sigma bond between Sand F in sulfur hexafluoride, SF6? orbital on S+ orbital on s o orbital on F rbital on F What are the approximate F-S-F bond angles ? (list all possible) 90...
Sigma Bonding A a bond arises from the straight-on overlap of two atomic orbitals. The electron...
 Sigma Bonding A a bond arises from the straight-on overlap of two atomic orbitals. The electron density lies Sigma Bonding along the axis of the two bonded nuclei sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 s orbital What atomic or hybrid orbital on Br makes up the sigma bond between Br and Cl in bromine trichloride, BrCl3? orbital on Br What are the approximate C-Br-Cl bond angles? (ist all possible separated by a space)
Sigma Bonding A a bond arises from the straight-on overlap of two atomic orbitals. The electron density lies Sigma Bonding along the axis of the two bonded nuclei sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 s orbital What atomic or hybrid orbital on Br makes up the sigma bond between Br and Cl in bromine trichloride, BrCl3? orbital on Br What are the approximate C-Br-Cl bond angles? (ist all possible separated by a space)
Sigma Bonding As bond arises from the straight-on overlap of two atomic orbitals. The electron density...
 Sigma Bonding As bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. p ridorbital Example: Sigma Bonding in methane, CHA 11 orbital What atomic or hybrid orbital on O makes up the sigma bond between 0 and H in water, H,0? orbital on O What is the approximate H-O-H bond angle? ant values if needed for this question Sigma Bonding Ac bond arises from the straight-on overlap...
Sigma Bonding As bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. p ridorbital Example: Sigma Bonding in methane, CHA 11 orbital What atomic or hybrid orbital on O makes up the sigma bond between 0 and H in water, H,0? orbital on O What is the approximate H-O-H bond angle? ant values if needed for this question Sigma Bonding Ac bond arises from the straight-on overlap...
help with this! Complete the Lewis structure of the compound shown below and indicate which of...
 help with this!
Complete the Lewis structure of the compound shown below and indicate which of the following statements are true. H false An sp2 hybrid orbital on C-1 overlaps with an sp hybrid orbital on C-2 to form the sigma bond between C-1 and C-2 false The C-N bond is formed from overlap of an sp? hybrid orbital from the carbon atom with an sp3 hybrid orbital from the nitrogen atom. true There are twelve o bonds in this...
help with this!
Complete the Lewis structure of the compound shown below and indicate which of the following statements are true. H false An sp2 hybrid orbital on C-1 overlaps with an sp hybrid orbital on C-2 to form the sigma bond between C-1 and C-2 false The C-N bond is formed from overlap of an sp? hybrid orbital from the carbon atom with an sp3 hybrid orbital from the nitrogen atom. true There are twelve o bonds in this...
Bonding An bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies...
 Bonding An bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals. p-orbital p-orbital atomi atom? it bond What atomic or hybrid orbitals make up the it bond between C and C2 in propyne, CHCCH3 ? orbital on Ci + orbital on C2 How many o bonds does C have in CHCCH3 ? How many it bonds does Chave? Bonding At...
Bonding An bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals. p-orbital p-orbital atomi atom? it bond What atomic or hybrid orbitals make up the it bond between C and C2 in propyne, CHCCH3 ? orbital on Ci + orbital on C2 How many o bonds does C have in CHCCH3 ? How many it bonds does Chave? Bonding At...
Sigma Bonding Ach Siamo Bonding A o bond arises from the straight-on overlap of two atomic...
 Sigma Bonding Ach Siamo Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between Brand F in bromine pentafluoride, BrFs? orbital on Br + orbital on F What are the approximate F.Br. F bond angles ? (list all possible) Bonding At bond arises...
Sigma Bonding Ach Siamo Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between Brand F in bromine pentafluoride, BrFs? orbital on Br + orbital on F What are the approximate F.Br. F bond angles ? (list all possible) Bonding At bond arises...
A Tt bond arises from "sideways" overlap of two parallel p orbitals. The electron In Bonding...
 A Tt bond arises from "sideways" overlap of two parallel p orbitals. The electron In Bonding density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals p-orbital p-orbitalt bond atomi atom What atomic or hybrid orbitals make up the t bond between N and O in nitrosyl chloride, NOCI? orbital on N+orbital on How many σ bonds does N have in NOCI ? | How many T bonds does N have ? Submit...
A Tt bond arises from "sideways" overlap of two parallel p orbitals. The electron In Bonding density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals p-orbital p-orbitalt bond atomi atom What atomic or hybrid orbitals make up the t bond between N and O in nitrosyl chloride, NOCI? orbital on N+orbital on How many σ bonds does N have in NOCI ? | How many T bonds does N have ? Submit...
please answer following question. (d) Cah 2. The molecule NO2 is a reactive gas with a...
 please answer
following question.
(d) Cah 2. The molecule NO2 is a reactive gas with a bond angle of 134.3º. (a) Draw the most stable possible Lewis structure(s) of NO2. (b) Determine the correct point group for NO2 and determine the sand p orbital symmetry Mulliken symbols for the central nitrogen from the character table. Make sure your molecule is in the xz plane with the z-axis as the main rotation axis. (©) Find the reducible representations for SALCs formed...
please answer
following question.
(d) Cah 2. The molecule NO2 is a reactive gas with a bond angle of 134.3º. (a) Draw the most stable possible Lewis structure(s) of NO2. (b) Determine the correct point group for NO2 and determine the sand p orbital symmetry Mulliken symbols for the central nitrogen from the character table. Make sure your molecule is in the xz plane with the z-axis as the main rotation axis. (©) Find the reducible representations for SALCs formed...
Most questions answered within 3 hours.
-
Calcium dihydrogen phosphate, Ca(H2PO4)2, and sodium hydrogen
carbonate, NaHCO3, are ingredients of baking powder that react...
asked 1 minute from now -
Design and implement your own linked list class to hold a sorted
list of integers in...
asked 13 minutes ago -
can you identify some rules of how R is calculated for a circuit
in series? For...
asked 5 minutes ago -
Write the balanced chemical equation for the reaction between
iodate and bisulfite. Does the order with...
asked 7 minutes ago -
Swissie Triangular Arbitrage. The following exchange rates are
available to you. (You can buy or sell...
asked 12 minutes ago -
a car starts from rest at a stop sign. It accelerates
at 4.4m/s² for 7.5s, coasts...
asked 13 minutes ago -
1. Sketch the expected Mass Spectrum of Pentane (C5H12)
2. Draw the most stable Newman projection...
asked 17 minutes ago -
There are changes in the dynamics of a relationship of a family
when family takes on...
asked 20 minutes ago -
Prove that the following is a valid cumulative distribution
function
F(x) = x/(1+x) for a>=0
0...
asked 19 minutes ago -
Caculate the PH THANK YOU!
[OH−] = 1.9×10−7 M
[OH−] = 1.6×10−8 M
[OH−] = 7.2×10−11...
asked 31 minutes ago -
Suppose you drive an old truck with a 32 gallon gas tank that
gets 16 miles/gallon....
asked 33 minutes ago -
What can be the anticipated results of curing diabetes with stem
cells?
asked 29 minutes ago
 Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbital on the central O atom makes up the sigma bond between this O and an outer H atom in water, H,O? orbital on o What is the approximate H-O-H bond angle? Sigma Bonding A o bond arises from...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbital on the central O atom makes up the sigma bond between this O and an outer H atom in water, H,O? orbital on o What is the approximate H-O-H bond angle? Sigma Bonding A o bond arises from...
 (e)
a- use of s and p atomic orbitals
b- use of s, p, and d atomic orbitals
c-use of only s orbitals
d-generation of more than four hybrid orbitals
Hybrid Orbitals Use the animation to answer the following questions. (a) Mixing yellow and blue atomic orbitals can produce: 2, 3 or 4 hybrid orbitals. O 4 or 5 hybrid orbitals. 1 or 2 hybrid orbital(s). O 5 or 6 hybrid orbitals. (b) Mixing one yellow atomic orbital and one...
(e)
a- use of s and p atomic orbitals
b- use of s, p, and d atomic orbitals
c-use of only s orbitals
d-generation of more than four hybrid orbitals
Hybrid Orbitals Use the animation to answer the following questions. (a) Mixing yellow and blue atomic orbitals can produce: 2, 3 or 4 hybrid orbitals. O 4 or 5 hybrid orbitals. 1 or 2 hybrid orbital(s). O 5 or 6 hybrid orbitals. (b) Mixing one yellow atomic orbital and one...
 Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density 5 lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbitals make up the sigma bond between Sand F in sulfur hexafluoride, SF6? orbital on S+ orbital on s o orbital on F rbital on F What are the approximate F-S-F bond angles ? (list all possible) 90...
Sigma Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density 5 lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH Is orbital What atomic or hybrid orbitals make up the sigma bond between Sand F in sulfur hexafluoride, SF6? orbital on S+ orbital on s o orbital on F rbital on F What are the approximate F-S-F bond angles ? (list all possible) 90...
 Sigma Bonding A a bond arises from the straight-on overlap of two atomic orbitals. The electron density lies Sigma Bonding along the axis of the two bonded nuclei sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 s orbital What atomic or hybrid orbital on Br makes up the sigma bond between Br and Cl in bromine trichloride, BrCl3? orbital on Br What are the approximate C-Br-Cl bond angles? (ist all possible separated by a space)
Sigma Bonding A a bond arises from the straight-on overlap of two atomic orbitals. The electron density lies Sigma Bonding along the axis of the two bonded nuclei sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 s orbital What atomic or hybrid orbital on Br makes up the sigma bond between Br and Cl in bromine trichloride, BrCl3? orbital on Br What are the approximate C-Br-Cl bond angles? (ist all possible separated by a space)
 Sigma Bonding As bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. p ridorbital Example: Sigma Bonding in methane, CHA 11 orbital What atomic or hybrid orbital on O makes up the sigma bond between 0 and H in water, H,0? orbital on O What is the approximate H-O-H bond angle? ant values if needed for this question Sigma Bonding Ac bond arises from the straight-on overlap...
Sigma Bonding As bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. p ridorbital Example: Sigma Bonding in methane, CHA 11 orbital What atomic or hybrid orbital on O makes up the sigma bond between 0 and H in water, H,0? orbital on O What is the approximate H-O-H bond angle? ant values if needed for this question Sigma Bonding Ac bond arises from the straight-on overlap...
 help with this!
Complete the Lewis structure of the compound shown below and indicate which of the following statements are true. H false An sp2 hybrid orbital on C-1 overlaps with an sp hybrid orbital on C-2 to form the sigma bond between C-1 and C-2 false The C-N bond is formed from overlap of an sp? hybrid orbital from the carbon atom with an sp3 hybrid orbital from the nitrogen atom. true There are twelve o bonds in this...
help with this!
Complete the Lewis structure of the compound shown below and indicate which of the following statements are true. H false An sp2 hybrid orbital on C-1 overlaps with an sp hybrid orbital on C-2 to form the sigma bond between C-1 and C-2 false The C-N bond is formed from overlap of an sp? hybrid orbital from the carbon atom with an sp3 hybrid orbital from the nitrogen atom. true There are twelve o bonds in this...
 Bonding An bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals. p-orbital p-orbital atomi atom? it bond What atomic or hybrid orbitals make up the it bond between C and C2 in propyne, CHCCH3 ? orbital on Ci + orbital on C2 How many o bonds does C have in CHCCH3 ? How many it bonds does Chave? Bonding At...
Bonding An bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals. p-orbital p-orbital atomi atom? it bond What atomic or hybrid orbitals make up the it bond between C and C2 in propyne, CHCCH3 ? orbital on Ci + orbital on C2 How many o bonds does C have in CHCCH3 ? How many it bonds does Chave? Bonding At...
 Sigma Bonding Ach Siamo Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between Brand F in bromine pentafluoride, BrFs? orbital on Br + orbital on F What are the approximate F.Br. F bond angles ? (list all possible) Bonding At bond arises...
Sigma Bonding Ach Siamo Bonding A o bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between Brand F in bromine pentafluoride, BrFs? orbital on Br + orbital on F What are the approximate F.Br. F bond angles ? (list all possible) Bonding At bond arises...
 A Tt bond arises from "sideways" overlap of two parallel p orbitals. The electron In Bonding density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals p-orbital p-orbitalt bond atomi atom What atomic or hybrid orbitals make up the t bond between N and O in nitrosyl chloride, NOCI? orbital on N+orbital on How many σ bonds does N have in NOCI ? | How many T bonds does N have ? Submit...
A Tt bond arises from "sideways" overlap of two parallel p orbitals. The electron In Bonding density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals p-orbital p-orbitalt bond atomi atom What atomic or hybrid orbitals make up the t bond between N and O in nitrosyl chloride, NOCI? orbital on N+orbital on How many σ bonds does N have in NOCI ? | How many T bonds does N have ? Submit...
 please answer
following question.
(d) Cah 2. The molecule NO2 is a reactive gas with a bond angle of 134.3º. (a) Draw the most stable possible Lewis structure(s) of NO2. (b) Determine the correct point group for NO2 and determine the sand p orbital symmetry Mulliken symbols for the central nitrogen from the character table. Make sure your molecule is in the xz plane with the z-axis as the main rotation axis. (©) Find the reducible representations for SALCs formed...
please answer
following question.
(d) Cah 2. The molecule NO2 is a reactive gas with a bond angle of 134.3º. (a) Draw the most stable possible Lewis structure(s) of NO2. (b) Determine the correct point group for NO2 and determine the sand p orbital symmetry Mulliken symbols for the central nitrogen from the character table. Make sure your molecule is in the xz plane with the z-axis as the main rotation axis. (©) Find the reducible representations for SALCs formed...