
A)How much mechanical work is required to operate the refrigerator for a cycle?
B)How much heat does the refrigerator discard to the high-temperature reservoir during each cycle?
Homework Answers


Add Answer to:
A)How much mechanical work is required to operate the
refrigerator for a cycle?
B)How much heat...
A refrigerator with a coefficient of performance of 1.80 absorbs 3.15×104 J of heat from the...
A refrigerator with a coefficient of performance of 1.80 absorbs 3.15×104 J of heat from the low-temperature reservoir during each cycle. How much mechanical work is required to operate the refrigerator for a cycle? How much heat does the refrigerator discard to the high-temperature reservoir during each cycle?
Problem 18.56 6 of 8 Review A refrigerator with a coefficient of performance of 1.85 absorbs...
 Problem 18.56 6 of 8 Review A refrigerator with a coefficient of performance of 1.85 absorbs 3.54x10* J of heat from the low- temperature reservoir during each cycle. Part A How much mechanical work is required to operate the refrigerator for a cycle? ΡΕΙ ΑΣφ + ? Submit Request Answer Part 8 How much heat does the refrigerator discard to the high-temperature reservoir during each cycle? 10 AED + + ? KJ Submit Request Answer
Problem 18.56 6 of 8 Review A refrigerator with a coefficient of performance of 1.85 absorbs 3.54x10* J of heat from the low- temperature reservoir during each cycle. Part A How much mechanical work is required to operate the refrigerator for a cycle? ΡΕΙ ΑΣφ + ? Submit Request Answer Part 8 How much heat does the refrigerator discard to the high-temperature reservoir during each cycle? 10 AED + + ? KJ Submit Request Answer
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir...
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
TIUVICU TU. A refrigerator has a coefficient of performance of K = 2.1. Each cycle, it...
 TIUVICU TU. A refrigerator has a coefficient of performance of K = 2.1. Each cycle, it absorbs 3.75x104 J of heat from the cold reservoir. The refrigerator is driven by a Carnot engine that has an efficiency of e=0.5 Eckboard - The Cit x Bb Pre-Lab Assignments - 201 X C Six HepID=9ca21d320b455f2dffd7601af0f84482#10001 Part A How much mechanical energy is required each cycle to operate the refrigerator? Express your answer in joules to two significant figures. IVO ALDO a ?...
TIUVICU TU. A refrigerator has a coefficient of performance of K = 2.1. Each cycle, it absorbs 3.75x104 J of heat from the cold reservoir. The refrigerator is driven by a Carnot engine that has an efficiency of e=0.5 Eckboard - The Cit x Bb Pre-Lab Assignments - 201 X C Six HepID=9ca21d320b455f2dffd7601af0f84482#10001 Part A How much mechanical energy is required each cycle to operate the refrigerator? Express your answer in joules to two significant figures. IVO ALDO a ?...
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
A refrigerator has a coefficient of performance of 1.15, and it extracts 7.95 J of heat...
A refrigerator has a coefficient of performance of 1.15, and it extracts 7.95 J of heat from the cold reservoir during each cycle. (a) How much work is done on the gas in each cycle? (b) How much heat is exhausted into the hot reservoir in each cycle? I've seen a similar question, but I still don't understand how to solve. Can I get help please, step by step would be kind.
An ideal refrigerator does 105.0 J of work to remove 555.0 J of heat from its...
An ideal refrigerator does 105.0 J of work to remove 555.0 J of heat from its cold compartment during each cycle. What is the refrigerator's coefficient of performance? Tries 0/12 How much heat per cycle is exhausted to the kitchen?
37. A refrigerator uses 40 J of work to extract heat from a heat reservoir at 0.00°C....
37. A refrigerator uses 40 J of work to extract heat from a heat reservoir at 0.00°C. If the coefficient of performance of the refrigerator is 2.1, then how much heat is extracted from the heat reservoir at 0.00°C? 90 J 84 J 48 J 65 J
ed to a Carnot refrigerator so that all of the work produced by the engine is...
 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
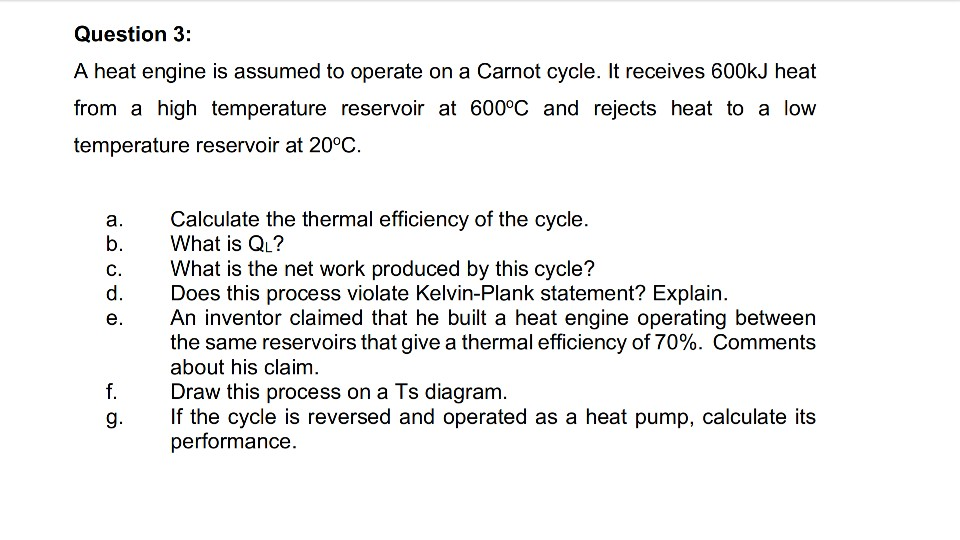 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Most questions answered within 3 hours.
-
1-Write an algorithm to get two numbers from the user (as
inputs) and calculate the sum...
asked 28 minutes ago -
Define white-collar crime. What is the difference between
offender and offense-based definitions of white-collar crime? What...
asked 1 hour ago -
Consider a reaction which is 1st order with respect to A and 1st
order with respect...
asked 1 hour ago -
c++
The length of the hypotenuse of a right-angled triangle is the
square root of the...
asked 1 hour ago -
When a metal rod is heated, not only its resistance but also its
length and cross‐sectional...
asked 1 hour ago -
write a c++ program that computes the L^1 - Norm of a given
vector (L^1 norm...
asked 1 hour ago -
A manufacturer of banana chips would like to know whether its
bag filling machine works correctly...
asked 1 hour ago -
Complete the chapter case, "Turnover Analysis".
Chapter Case
Turnover Analysis
You recently completed your company’s new...
asked 1 hour ago -
What is the pH of solutions having the following H3O+
concentrations? Identify each as acidic, basic,...
asked 1 hour ago -
How does over-voltage of the diode usually occur?
asked 2 hours ago -
If you step on a dirty nail, your doctor will recommend you
update your Tetanus vaccination....
asked 2 hours ago -
Suppose that decreases in the price of milk lead to decrease s
in the retail prices...
asked 2 hours ago
 Problem 18.56 6 of 8 Review A refrigerator with a coefficient of performance of 1.85 absorbs 3.54x10* J of heat from the low- temperature reservoir during each cycle. Part A How much mechanical work is required to operate the refrigerator for a cycle? ΡΕΙ ΑΣφ + ? Submit Request Answer Part 8 How much heat does the refrigerator discard to the high-temperature reservoir during each cycle? 10 AED + + ? KJ Submit Request Answer
Problem 18.56 6 of 8 Review A refrigerator with a coefficient of performance of 1.85 absorbs 3.54x10* J of heat from the low- temperature reservoir during each cycle. Part A How much mechanical work is required to operate the refrigerator for a cycle? ΡΕΙ ΑΣφ + ? Submit Request Answer Part 8 How much heat does the refrigerator discard to the high-temperature reservoir during each cycle? 10 AED + + ? KJ Submit Request Answer
 (a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
(a) During each cycle, a Carnot engine absorbs 772 J as heat from a high-temperature reservoir at 388 K, with the low-temperature reservoir at 287 K. How much work is done per cycle? (b) The engine is then made to work in reverse to function as a Carnot refrigerator between those same two reservoirs. During each cycle, how much work is required to remove 1206 J as heat from the low-temperature reservoir? () Numbel 200.9590 UnitsT j UnitsT j (b)...
 TIUVICU TU. A refrigerator has a coefficient of performance of K = 2.1. Each cycle, it absorbs 3.75x104 J of heat from the cold reservoir. The refrigerator is driven by a Carnot engine that has an efficiency of e=0.5 Eckboard - The Cit x Bb Pre-Lab Assignments - 201 X C Six HepID=9ca21d320b455f2dffd7601af0f84482#10001 Part A How much mechanical energy is required each cycle to operate the refrigerator? Express your answer in joules to two significant figures. IVO ALDO a ?...
TIUVICU TU. A refrigerator has a coefficient of performance of K = 2.1. Each cycle, it absorbs 3.75x104 J of heat from the cold reservoir. The refrigerator is driven by a Carnot engine that has an efficiency of e=0.5 Eckboard - The Cit x Bb Pre-Lab Assignments - 201 X C Six HepID=9ca21d320b455f2dffd7601af0f84482#10001 Part A How much mechanical energy is required each cycle to operate the refrigerator? Express your answer in joules to two significant figures. IVO ALDO a ?...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...