
Homework Answers

Add Answer to:
please show work. i need to know how to do this for a
test
A flask...
A flask is charged with 0.124 mol of A and allowed to react to form B...
 A flask is charged with 0.124 mol of A and allowed
to react to form B according to the reaction A(g)
B(g). The following data are obtained
for A as the reaction proceeds:
The average rate of disappearance of A between 20.0 s and 40.0 s
is ________ mol/s
Select one:
A. 1.4 × 10–3
B. 7.1 × 10–3
C. 8.5 × 10–4
D. 1.7 × 10–3
E. 590
Time (S) 0.0 10.0 20.0 30.0 40.0 Moles of A 0.124...
A flask is charged with 0.124 mol of A and allowed
to react to form B according to the reaction A(g)
B(g). The following data are obtained
for A as the reaction proceeds:
The average rate of disappearance of A between 20.0 s and 40.0 s
is ________ mol/s
Select one:
A. 1.4 × 10–3
B. 7.1 × 10–3
C. 8.5 × 10–4
D. 1.7 × 10–3
E. 590
Time (S) 0.0 10.0 20.0 30.0 40.0 Moles of A 0.124...
Hi, it is one question with parts (a,b,c,d) also complete the table
 Hi, it is one question with parts (a,b,c,d)
also complete the table
2. A flask is charged with 1.000 mol of A and allowed to react to form B according to the reaction A (g) 2B (g). The following data is obtained for [A] as the reaction proceeds. (10) Complete the table. 0,0 100 200 300 400 Time 0.00 20.0 30.0 40.0 min mol mol 0.835 0.714 0.625 0.555 1.000 [Bl Disappearance of A Appearance of B mol min Avg.rate...
Hi, it is one question with parts (a,b,c,d)
also complete the table
2. A flask is charged with 1.000 mol of A and allowed to react to form B according to the reaction A (g) 2B (g). The following data is obtained for [A] as the reaction proceeds. (10) Complete the table. 0,0 100 200 300 400 Time 0.00 20.0 30.0 40.0 min mol mol 0.835 0.714 0.625 0.555 1.000 [Bl Disappearance of A Appearance of B mol min Avg.rate...
A flask is charged with 2.78 mol of A and allowed to react to form B...
 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) mols of A 0.0 2.78 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 The average rate of disappearance of A for the first 30s is _ mol/s. *Please report 2 significant figures. Numbers only, no unit. No scientific notation. The value...
A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) mols of A 0.0 2.78 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 The average rate of disappearance of A for the first 30s is _ mol/s. *Please report 2 significant figures. Numbers only, no unit. No scientific notation. The value...
please show all work i need to know how to do this for a test The...
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please help with these two QUESTION 20 Which of the reactions will have the largest rate...
 please help with these two
QUESTION 20 Which of the reactions will have the largest rate constant? oc OD OA More information is required. QUESTION 21 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(8) -B(8). The following data are obtained for [A] as the reaction proceeds: 0.0 Time (s) mols of A 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 2.78 The average...
please help with these two
QUESTION 20 Which of the reactions will have the largest rate constant? oc OD OA More information is required. QUESTION 21 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(8) -B(8). The following data are obtained for [A] as the reaction proceeds: 0.0 Time (s) mols of A 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 2.78 The average...
1) Scientific Notation for first problem not necessary, only 3 sig figs needed. Please explain how...
 1)
Scientific Notation for first problem not necessary, only 3 sig
figs needed. Please explain how answer was obtained and box or
circle final answers.
2)
3)
Please explain how answer was obtained as I am having trouble
with third question
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a...
1)
Scientific Notation for first problem not necessary, only 3 sig
figs needed. Please explain how answer was obtained and box or
circle final answers.
2)
3)
Please explain how answer was obtained as I am having trouble
with third question
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a...
1) 2) 3) At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction:...
 1)
2)
3)
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a 2.0-L flask? The decomposition of dinitrogen tetroxide to nitrogen dioxide at 400°C follows first-order kinetics with a rate constant of 2.86 x10-35-1. Starting with pure N204, how many minutes will it take for 80.0% to decompose? *Please report...
1)
2)
3)
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a 2.0-L flask? The decomposition of dinitrogen tetroxide to nitrogen dioxide at 400°C follows first-order kinetics with a rate constant of 2.86 x10-35-1. Starting with pure N204, how many minutes will it take for 80.0% to decompose? *Please report...
please show work i need to know this for a test Question 20 1 pts The...
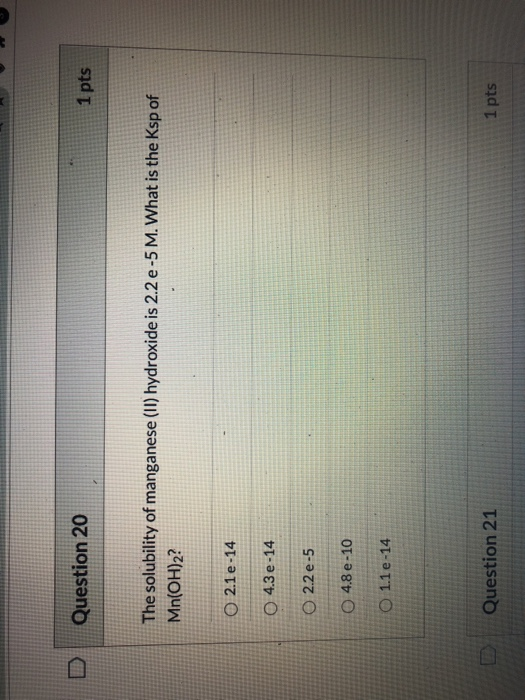 please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work. i need to know how to do this for a test Question 18...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
I don't understand how to do these. Please help, thank you! LAB VIL. PRELAB EXERCISE Name...
 I don't understand how to do these. Please help, thank you!
LAB VIL. PRELAB EXERCISE Name Section Consider the reaction, SO+32so+ Is. Data obtained in measuring rate of formation of Is are listed in the table. II1, M Initial rate, Ms 0.035 0.070 0.070 0.055 0.055 0.110 15 x 10 3.0 x 10 6.0 x 10 I. Determine the order of reaction with respect to S:Os 2What is the onder of reactin with reset t 3. Give the overall order...
I don't understand how to do these. Please help, thank you!
LAB VIL. PRELAB EXERCISE Name Section Consider the reaction, SO+32so+ Is. Data obtained in measuring rate of formation of Is are listed in the table. II1, M Initial rate, Ms 0.035 0.070 0.070 0.055 0.055 0.110 15 x 10 3.0 x 10 6.0 x 10 I. Determine the order of reaction with respect to S:Os 2What is the onder of reactin with reset t 3. Give the overall order...
Most questions answered within 3 hours.
-
Consider a 1.0 L buffer containing 0.092 mol L-1 HCOOH and 0.100
mol L-1 HCOO-. What...
asked 4 minutes ago -
Koch Realty has owned a vacant land with a FMV of
$775,000 and an adjusted basis...
asked 10 minutes ago -
It is estimated 29% of all adults in United States invest in
stocks and that 85%...
asked 10 minutes ago -
What does a 2-sided p value of 0.04 mean? (I am not asking if it
is...
asked 24 minutes ago -
A parallel-plate capacitor is made from two aluminum-foil
sheets, each 7.8 cmcm wide and 5.1 mmlong....
asked 25 minutes ago -
1. why is toluene a stronger nucleophile than benzene?
2.why is phenol a stronger nucleophile than...
asked 42 minutes ago -
4. How can you solve for the density of the liquid from the
slope? Please show...
asked 42 minutes ago -
when 2053 j of heat is added to 46.3 g of hexane C6H14 the
temperature increases...
asked 1 hour ago -
I need new and unique answers, please. (Use your own words,
don't copy and paste), Please...
asked 1 hour ago -
MCL 445.111 et seq. deals with Home Solicitation Sales.
MCL stands for Michigan Compiled Laws which...
asked 59 minutes ago -
Which of the following items may not create an NOL?
a.
sole proprietorship loss
b.
personal...
asked 1 hour ago -
A hypothetical solution forms between a solid and a liquid. The
values of the thermodynamic quantities...
asked 1 hour ago
 A flask is charged with 0.124 mol of A and allowed
to react to form B according to the reaction A(g)
B(g). The following data are obtained
for A as the reaction proceeds:
The average rate of disappearance of A between 20.0 s and 40.0 s
is ________ mol/s
Select one:
A. 1.4 × 10–3
B. 7.1 × 10–3
C. 8.5 × 10–4
D. 1.7 × 10–3
E. 590
Time (S) 0.0 10.0 20.0 30.0 40.0 Moles of A 0.124...
A flask is charged with 0.124 mol of A and allowed
to react to form B according to the reaction A(g)
B(g). The following data are obtained
for A as the reaction proceeds:
The average rate of disappearance of A between 20.0 s and 40.0 s
is ________ mol/s
Select one:
A. 1.4 × 10–3
B. 7.1 × 10–3
C. 8.5 × 10–4
D. 1.7 × 10–3
E. 590
Time (S) 0.0 10.0 20.0 30.0 40.0 Moles of A 0.124...
 Hi, it is one question with parts (a,b,c,d)
also complete the table
2. A flask is charged with 1.000 mol of A and allowed to react to form B according to the reaction A (g) 2B (g). The following data is obtained for [A] as the reaction proceeds. (10) Complete the table. 0,0 100 200 300 400 Time 0.00 20.0 30.0 40.0 min mol mol 0.835 0.714 0.625 0.555 1.000 [Bl Disappearance of A Appearance of B mol min Avg.rate...
Hi, it is one question with parts (a,b,c,d)
also complete the table
2. A flask is charged with 1.000 mol of A and allowed to react to form B according to the reaction A (g) 2B (g). The following data is obtained for [A] as the reaction proceeds. (10) Complete the table. 0,0 100 200 300 400 Time 0.00 20.0 30.0 40.0 min mol mol 0.835 0.714 0.625 0.555 1.000 [Bl Disappearance of A Appearance of B mol min Avg.rate...
 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) mols of A 0.0 2.78 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 The average rate of disappearance of A for the first 30s is _ mol/s. *Please report 2 significant figures. Numbers only, no unit. No scientific notation. The value...
A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) mols of A 0.0 2.78 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 The average rate of disappearance of A for the first 30s is _ mol/s. *Please report 2 significant figures. Numbers only, no unit. No scientific notation. The value...
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
 please help with these two
QUESTION 20 Which of the reactions will have the largest rate constant? oc OD OA More information is required. QUESTION 21 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(8) -B(8). The following data are obtained for [A] as the reaction proceeds: 0.0 Time (s) mols of A 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 2.78 The average...
please help with these two
QUESTION 20 Which of the reactions will have the largest rate constant? oc OD OA More information is required. QUESTION 21 A flask is charged with 2.78 mol of A and allowed to react to form B according to the reaction A(8) -B(8). The following data are obtained for [A] as the reaction proceeds: 0.0 Time (s) mols of A 10.0 2.43 20.0 2.18 30.0 2.02 40.0 1.89 50.0 1.70 60.0 1.58 2.78 The average...
 1)
Scientific Notation for first problem not necessary, only 3 sig
figs needed. Please explain how answer was obtained and box or
circle final answers.
2)
3)
Please explain how answer was obtained as I am having trouble
with third question
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a...
1)
Scientific Notation for first problem not necessary, only 3 sig
figs needed. Please explain how answer was obtained and box or
circle final answers.
2)
3)
Please explain how answer was obtained as I am having trouble
with third question
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a...
 1)
2)
3)
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a 2.0-L flask? The decomposition of dinitrogen tetroxide to nitrogen dioxide at 400°C follows first-order kinetics with a rate constant of 2.86 x10-35-1. Starting with pure N204, how many minutes will it take for 80.0% to decompose? *Please report...
1)
2)
3)
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction: 2 IC1(g)=12(8) + Cl2(g). What is the equilibrium concentration of Cl2 if 3.18 mol of I2 and 3.18 mol of Cl2 are initially mixed in a 2.0-L flask? The decomposition of dinitrogen tetroxide to nitrogen dioxide at 400°C follows first-order kinetics with a rate constant of 2.86 x10-35-1. Starting with pure N204, how many minutes will it take for 80.0% to decompose? *Please report...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
 I don't understand how to do these. Please help, thank you!
LAB VIL. PRELAB EXERCISE Name Section Consider the reaction, SO+32so+ Is. Data obtained in measuring rate of formation of Is are listed in the table. II1, M Initial rate, Ms 0.035 0.070 0.070 0.055 0.055 0.110 15 x 10 3.0 x 10 6.0 x 10 I. Determine the order of reaction with respect to S:Os 2What is the onder of reactin with reset t 3. Give the overall order...
I don't understand how to do these. Please help, thank you!
LAB VIL. PRELAB EXERCISE Name Section Consider the reaction, SO+32so+ Is. Data obtained in measuring rate of formation of Is are listed in the table. II1, M Initial rate, Ms 0.035 0.070 0.070 0.055 0.055 0.110 15 x 10 3.0 x 10 6.0 x 10 I. Determine the order of reaction with respect to S:Os 2What is the onder of reactin with reset t 3. Give the overall order...