![The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.](http://img.homeworklib.com/questions/af210800-0bb4-11eb-a1e3-97b515131366.png?x-oss-process=image/resize,w_560)
Homework Answers
For the first order reaction
ln(A0/At) = kt
We can see that the concentration drops from 0.20M to 0.10M in 10 seconds, hence the half-life is 10 seconds. After 40 seconds, it will be 4 half lifes
Concentration after 40 seconds = 0.20/2^4 = 0.0125 (hence the correct answer is 1.3* 10^(-2) or 1.3 e-2)
First option is correct answer.
Note - Post any doubts/queries in comments section.
Add Answer to:
please show all work i need to know how to do this for a
test
The...
* Please show work step by step explaining how you got to the solution* Consider the...
* Please show work step by step explaining how you got to the solution* Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 Graph the [A] vs. time, ln[A] vs time and 1/[A] vs. time. Determine the rate law and rate constant (including units) for the reaction. Determine the time at which the concentration of A at will be 0.12 M.
please show work. i need this for a test Question 21 1 pts The reaction AB...
 please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
please show work. i need to know how to do this for a test A flask...
 please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show work. i need to know how to do this for a test Question 18...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
6) is the 102 -1 at 250 °C. What 6) A particular first-order reaction has a...
 6) is the 102 -1 at 250 °C. What 6) A particular first-order reaction has a rate constant of 1.35 magnitude of k at 35.0°C if Ea - 55.5 kJ/mol? A) 1.93 x 1035 B) 1.35 x 102 C) 1.05 x 104 D) 98 E) 2.80 x 102 The reaction A - B is first order in (A). Consider the following data. Time (s) [A] (M) 0.0 0.20 5.0 0.14 10.0 0.10 15.0 0.071 20.0 0.040 7) - 7) What...
6) is the 102 -1 at 250 °C. What 6) A particular first-order reaction has a rate constant of 1.35 magnitude of k at 35.0°C if Ea - 55.5 kJ/mol? A) 1.93 x 1035 B) 1.35 x 102 C) 1.05 x 104 D) 98 E) 2.80 x 102 The reaction A - B is first order in (A). Consider the following data. Time (s) [A] (M) 0.0 0.20 5.0 0.14 10.0 0.10 15.0 0.071 20.0 0.040 7) - 7) What...
please show work i need to know this for a test Question 20 1 pts The...
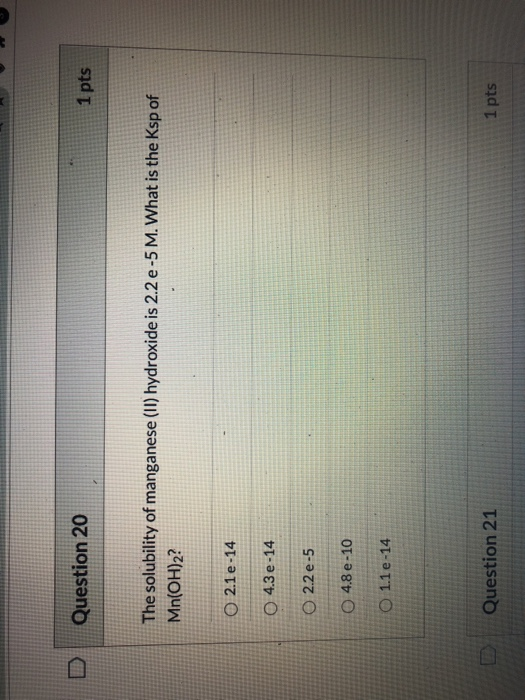 please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show all work. i need to know process for test A compound decomposes by a...
 please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
Show work please. So I know how to apporach problems like these in the future. (2...
 Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
Answer all assigned questions and problems, and show all work. Determine the pH of (a) a...
Answer all assigned questions and problems, and show all work. Determine the pH of (a) a 0.40 MCH3CO2H solution, (b) a solution that is 0.40 MCH3CO2H and 0.20 MNaCH3CO2. (8 points) (Reference: Chang 16.5) Which of the following solutions can act as a buffer? (a) KCl/HCl, (b) KHSO4/H2SO4, (c) KNO2/HNO2.(5 points) (Reference: Chang 16.9) Calculate the pH of the buffer system made up of 0.15 MNH3/0.35 MNH4Cl. (5 points) (Reference: Chang 16.11) The pH of a bicarbonate-carbonic acid buffer is...
please help solve so i may know how to do on test Ratino, is evaluating an...
 please help solve so i may know how to do on test
Ratino, is evaluating an investment proposal that will require an initial outlay of $804,000 and would yield yearly cash inflows of $200,000 for nine years. The company uses a discount rate of 10%. What is the NPV of the investment? Select one O A $350,000 O B . 3402.000 O C. 347.300 O D . 51.151.000 o E. None of the above
please help solve so i may know how to do on test
Ratino, is evaluating an investment proposal that will require an initial outlay of $804,000 and would yield yearly cash inflows of $200,000 for nine years. The company uses a discount rate of 10%. What is the NPV of the investment? Select one O A $350,000 O B . 3402.000 O C. 347.300 O D . 51.151.000 o E. None of the above
Most questions answered within 3 hours.
-
a
dairy farmer notices that a citcular water trough near the barn has
become rusty with...
asked 14 minutes ago -
In a study of the accuracy of fast food drive-through orders,
Restaurant A had 283 accurate...
asked 16 minutes ago -
A share of common stock has just paid a dividend of $2.50. If
the expected long-run...
asked 25 minutes ago -
1. List all actors whose last names start
with letter "K"? The output needs to include the actor's...
asked 28 minutes ago -
1.The New England Merchants Bank Building in Boston is 152 m
high. On windy days it...
asked 31 minutes ago -
Can someone explain this code with comments I am supposed to dispay an array an add...
asked 45 minutes ago -
MKT2283 Sales Management Week 2 Assignment
Making Sales Management Decisions – Pronto Retail
Centers
Sales Management,...
asked 52 minutes ago -
Uranium is distributed in a form called yellow cake, which is
made from uranium ore. In...
asked 1 hour ago -
Python:
Write a func that will create a list of random numbers. The
function will take...
asked 1 hour ago -
Which of the following statements below is correct? There might
be more than one correct answer....
asked 1 hour ago -
You find that the annual standard deviation of a stock's returns
is equal to 35%. For...
asked 1 hour ago -
A 2,000 kg car is traveling at 20.0 m/s down a long mountain
grade of 1.00%....
asked 1 hour ago
 please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
 please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
 6) is the 102 -1 at 250 °C. What 6) A particular first-order reaction has a rate constant of 1.35 magnitude of k at 35.0°C if Ea - 55.5 kJ/mol? A) 1.93 x 1035 B) 1.35 x 102 C) 1.05 x 104 D) 98 E) 2.80 x 102 The reaction A - B is first order in (A). Consider the following data. Time (s) [A] (M) 0.0 0.20 5.0 0.14 10.0 0.10 15.0 0.071 20.0 0.040 7) - 7) What...
6) is the 102 -1 at 250 °C. What 6) A particular first-order reaction has a rate constant of 1.35 magnitude of k at 35.0°C if Ea - 55.5 kJ/mol? A) 1.93 x 1035 B) 1.35 x 102 C) 1.05 x 104 D) 98 E) 2.80 x 102 The reaction A - B is first order in (A). Consider the following data. Time (s) [A] (M) 0.0 0.20 5.0 0.14 10.0 0.10 15.0 0.071 20.0 0.040 7) - 7) What...
 please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
 Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
 please help solve so i may know how to do on test
Ratino, is evaluating an investment proposal that will require an initial outlay of $804,000 and would yield yearly cash inflows of $200,000 for nine years. The company uses a discount rate of 10%. What is the NPV of the investment? Select one O A $350,000 O B . 3402.000 O C. 347.300 O D . 51.151.000 o E. None of the above
please help solve so i may know how to do on test
Ratino, is evaluating an investment proposal that will require an initial outlay of $804,000 and would yield yearly cash inflows of $200,000 for nine years. The company uses a discount rate of 10%. What is the NPV of the investment? Select one O A $350,000 O B . 3402.000 O C. 347.300 O D . 51.151.000 o E. None of the above