![Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0](http://img.homeworklib.com/questions/0fe73660-384a-11ec-be1a-27074e9a8393.png?x-oss-process=image/resize,w_560)
Homework Answers
Question 21.
Solution:
Use the following equation to calculate the rate constant, k.
k = (2.303 / t)log([A]0 / [A]F) ---> (1)
t = 20 s, [A]0 = 2.3 M, [A]F = 0.14 M
Plug the values in (1) and solve.
k = (2.303 / 20 s)log{(2.3 M) / (0.14 M)}
k = (0.1152 s-1)log(16.4285)
k = (0.1152 s-1) (1.2156) = 0.14 s-1
Hence, 5th option is correct.
Add Answer to:
please show work. i need this for a test
Question 21 1 pts The reaction AB...
please show all work i need to know how to do this for a test The...
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
PLEASE SHOW ALL STEPS FOR COMPREHENSION. The reaction is the first order in [A]. Consider the...
 PLEASE SHOW ALL STEPS FOR
COMPREHENSION.
The reaction
is the first order in [A]. Consider the following data:
Time
[A] M
0.0
1.60
10.0
0.40
20.0
0.10
The rate constant for this experiment is ______
s-1?.
A – B
PLEASE SHOW ALL STEPS FOR
COMPREHENSION.
The reaction
is the first order in [A]. Consider the following data:
Time
[A] M
0.0
1.60
10.0
0.40
20.0
0.10
The rate constant for this experiment is ______
s-1?.
A – B
* Please show work step by step explaining how you got to the solution* Consider the...
* Please show work step by step explaining how you got to the solution* Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 Graph the [A] vs. time, ln[A] vs time and 1/[A] vs. time. Determine the rate law and rate constant (including units) for the reaction. Determine the time at which the concentration of A at will be 0.12 M.
please show work i need to know this for a test Question 20 1 pts The...
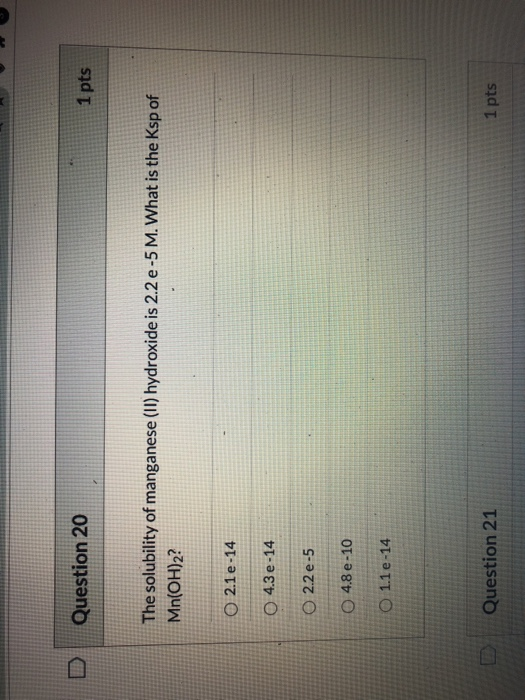 please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work. i need to know how to do this for a test Question 18...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a test A flask...
 please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show all work. i need to know process for test A compound decomposes by a...
 please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
Show work please. So I know how to apporach problems like these in the future. (2...
 Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
please show all work. i need this for a test Which of the following linear plots...
 please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
please show the work Consider the chemical reaction: NO + Cl2 → NOCI + CI This...
 please show the work
Consider the chemical reaction: NO + Cl2 → NOCI + CI This reaction is first order in each reactant and second order overall. To measure the rate constant, k, you decide to overload the amount of Cl2, at a concentration of 4 mol/L. The NO concentration used is much lower than this value. It is a good approximation to assume that the concentration of Cl2 does not change during the time that the reaction is studied,...
please show the work
Consider the chemical reaction: NO + Cl2 → NOCI + CI This reaction is first order in each reactant and second order overall. To measure the rate constant, k, you decide to overload the amount of Cl2, at a concentration of 4 mol/L. The NO concentration used is much lower than this value. It is a good approximation to assume that the concentration of Cl2 does not change during the time that the reaction is studied,...
Most questions answered within 3 hours.
-
1-Write an algorithm to get two numbers from the user (as
inputs) and calculate the sum...
asked 2 hours ago -
Define white-collar crime. What is the difference between
offender and offense-based definitions of white-collar crime? What...
asked 3 hours ago -
Consider a reaction which is 1st order with respect to A and 1st
order with respect...
asked 3 hours ago -
c++
The length of the hypotenuse of a right-angled triangle is the
square root of the...
asked 3 hours ago -
When a metal rod is heated, not only its resistance but also its
length and cross‐sectional...
asked 3 hours ago -
write a c++ program that computes the L^1 - Norm of a given
vector (L^1 norm...
asked 3 hours ago -
A manufacturer of banana chips would like to know whether its
bag filling machine works correctly...
asked 4 hours ago -
Complete the chapter case, "Turnover Analysis".
Chapter Case
Turnover Analysis
You recently completed your company’s new...
asked 4 hours ago -
What is the pH of solutions having the following H3O+
concentrations? Identify each as acidic, basic,...
asked 4 hours ago -
How does over-voltage of the diode usually occur?
asked 4 hours ago -
If you step on a dirty nail, your doctor will recommend you
update your Tetanus vaccination....
asked 4 hours ago -
Suppose that decreases in the price of milk lead to decrease s
in the retail prices...
asked 4 hours ago
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
 PLEASE SHOW ALL STEPS FOR
COMPREHENSION.
The reaction
is the first order in [A]. Consider the following data:
Time
[A] M
0.0
1.60
10.0
0.40
20.0
0.10
The rate constant for this experiment is ______
s-1?.
A – B
PLEASE SHOW ALL STEPS FOR
COMPREHENSION.
The reaction
is the first order in [A]. Consider the following data:
Time
[A] M
0.0
1.60
10.0
0.40
20.0
0.10
The rate constant for this experiment is ______
s-1?.
A – B
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
 please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
please show work. i need to know how to do this for a
test
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: Time (s) 0.00 10.0- 20.0 30.0 40.0 Moles of A 0.124 0.110 0.088 0.073 0.054 The average rate of disappearance of A between 10 s and 20 s is mol/s. ect Answer 2.2...
 please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
please show all work. i need to know process for test
A compound decomposes by a first-order process. If 60% of the compound remains after 60 minutes, the half-life of the compound is minutes. -5 O 44 0 -18 45 81
 Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
Show work please. So I know how to apporach problems like these in
the future.
(2 points) Determine the complete rate law for the following reaction using the data provided. 7. 2 NO(g) +O2(g)2 NO2(g) [NO]i (M) [O2]i (M) Initial Rate (M-Is-I) 0.030 0.030 0.060 0.00558.55 x 10-3 0.01101.71 x 10-2 0.0055 3.42×10-2 What is the initial rate if the concentration of both species is 0.050 M? (1 points) Consider the following reaction: A reaction mixture initially contains 2.24 atm...
 please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
 please show the work
Consider the chemical reaction: NO + Cl2 → NOCI + CI This reaction is first order in each reactant and second order overall. To measure the rate constant, k, you decide to overload the amount of Cl2, at a concentration of 4 mol/L. The NO concentration used is much lower than this value. It is a good approximation to assume that the concentration of Cl2 does not change during the time that the reaction is studied,...
please show the work
Consider the chemical reaction: NO + Cl2 → NOCI + CI This reaction is first order in each reactant and second order overall. To measure the rate constant, k, you decide to overload the amount of Cl2, at a concentration of 4 mol/L. The NO concentration used is much lower than this value. It is a good approximation to assume that the concentration of Cl2 does not change during the time that the reaction is studied,...