
Homework Answers
As we know that first order process:
ln(C/Co) = -kt
here C = The concentration of the remaining at time t;
Co =The initial concentration;
k = THe rate constant
As from the question it is given 60% decomposes therefore 40% remains
=>C/Co * 100% = 40% => C/Co = 0.40
ln(0.40) = -k * 60 =>-0.91 = -k*60
=>rate constant k = 0.0152 min-1
and the Half-life = ln(2)/k
= 0.6931/0.0152
= 45.59 ≈ 45 minutes
Add Answer to:
please show all work. i need to know process for test
A compound decomposes by a...
PROBLEMS. SHOW ALL WORK TO RECEIVE FULL CREDIT. NO WORK NO CREDIT 1)A compound decomposes by...
 PROBLEMS. SHOW ALL WORK TO RECEIVE FULL CREDIT. NO WORK NO CREDIT 1)A compound decomposes by a first-order process. If 21% of the compound decomposes in 60 minutes, calculate the half-life (in minutes) of the compound. (10 points) Ln CA- LnA-K +V2= Ln2 K 36005 0.211 0.79 -4.56-0.a36-K60min K.60mint 1.304 KEO.0aa063 +V2= Ln31.41 min 9
PROBLEMS. SHOW ALL WORK TO RECEIVE FULL CREDIT. NO WORK NO CREDIT 1)A compound decomposes by a first-order process. If 21% of the compound decomposes in 60 minutes, calculate the half-life (in minutes) of the compound. (10 points) Ln CA- LnA-K +V2= Ln2 K 36005 0.211 0.79 -4.56-0.a36-K60min K.60mint 1.304 KEO.0aa063 +V2= Ln31.41 min 9
a compound decomposes by a first order process . if 36% of the compound decomposes in...
a compound decomposes by a first order process . if 36% of the compound decomposes in 60 minutes , the half life pf the compound is ? 38 -5 41 -18 93
please show all work i need to know how to do this for a test The...
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show work. i need to know how to do this for a test Question 18...
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work i need to know this for a test Question 20 1 pts The...
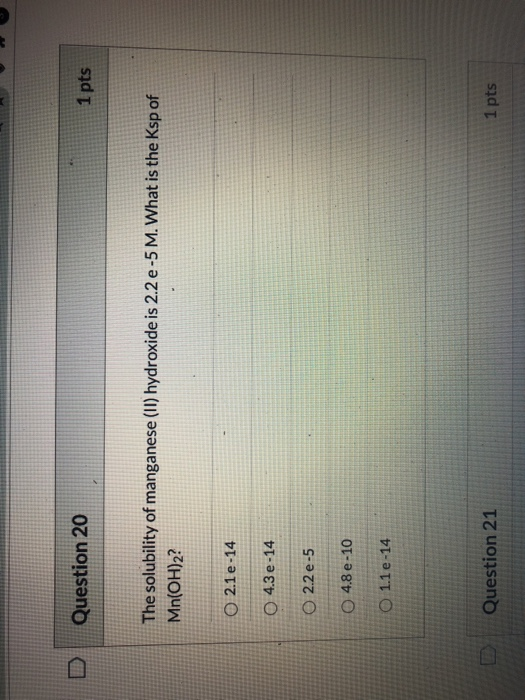 please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please show work i need to know this for a test
Question 20 1 pts The solubility of manganese (II) hydroxide is 2.2 e-5 M. What is the Ksp of Mn(OH)2? 0 2.1e-14 4.3 e-14 O 2.2 e-5 O 4.8 e -10 O 1.1 e -14 Question 21 1 pts
please help with question 25 a and b. showing all work 25. a. (3pts) A radioactive...
 please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass = 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k = 7.12 x 10 sec"!, what will the concentration...
please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass = 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k = 7.12 x 10 sec"!, what will the concentration...
please help with question 25 a and b. showing all work 25. a. (3pts) A radioactive...
 please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. On k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k 7.12x 10sec, what will the concentration be after 2...
please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. On k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k 7.12x 10sec, what will the concentration be after 2...
please show all work. i need this for a test Which of the following linear plots...
 please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
I know the final answer but I need to know all steps please show work for...
 I know the final answer but I need to know all steps please show
work for good rate
Problem 1. A manufacturer has a machine that, when operational at the beginning of a day, has a probability of 0.13 of breaking down sometime during the day. When this happens, the repair is done the next day and completed at the end of that day (a) Formulate the evolution of the status of the machine as a Markov chain by identifying...
I know the final answer but I need to know all steps please show
work for good rate
Problem 1. A manufacturer has a machine that, when operational at the beginning of a day, has a probability of 0.13 of breaking down sometime during the day. When this happens, the repair is done the next day and completed at the end of that day (a) Formulate the evolution of the status of the machine as a Markov chain by identifying...
please show work. i need this for a test Question 21 1 pts The reaction AB...
 please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
Most questions answered within 3 hours.
-
Humans have used horses for transportation for millions of
years. Therefore, they will use horses for...
asked 1 hour ago -
The following are the Jensen Corporation's unit costs of making
and selling an item at a...
asked 1 hour ago -
Does direct Medicare reimbursement of Advanced practice nurses
increase access to their services?
asked 2 hours ago -
List and explain why a company would choose to use a
published
compensation survey vs. creating...
asked 2 hours ago -
A discrete random variable X can take values from 1 to 10. Find
the variance of...
asked 2 hours ago -
The primary financial goal of a corporation is to maximize:
shareholders wealth.
earnings per share.
stock...
asked 2 hours ago -
determine whether the vectors u=(1,2,3,), v=(-2,1,0) and
w=(1,0,1) are linearly dependent or independent.
asked 3 hours ago -
python
Define a function called print_values which takes a dictionary
object as a parameter. The function...
asked 4 hours ago -
In Chapter 1 you created a program named Triangle in
which you displayed a seven-line triangle...
asked 4 hours ago -
Research question: What are the differences between separately
stated and non separately stated transactions in an...
asked 4 hours ago -
By using Arduino write a code that connects two LEDs to two
push-buttons. Each button controls...
asked 5 hours ago -
Bank of America has bonds that pay a coupon interest rate of 5.5
percent and mature...
asked 6 hours ago
 PROBLEMS. SHOW ALL WORK TO RECEIVE FULL CREDIT. NO WORK NO CREDIT 1)A compound decomposes by a first-order process. If 21% of the compound decomposes in 60 minutes, calculate the half-life (in minutes) of the compound. (10 points) Ln CA- LnA-K +V2= Ln2 K 36005 0.211 0.79 -4.56-0.a36-K60min K.60mint 1.304 KEO.0aa063 +V2= Ln31.41 min 9
PROBLEMS. SHOW ALL WORK TO RECEIVE FULL CREDIT. NO WORK NO CREDIT 1)A compound decomposes by a first-order process. If 21% of the compound decomposes in 60 minutes, calculate the half-life (in minutes) of the compound. (10 points) Ln CA- LnA-K +V2= Ln2 K 36005 0.211 0.79 -4.56-0.a36-K60min K.60mint 1.304 KEO.0aa063 +V2= Ln31.41 min 9
 please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
please show all work i need to know how to do this for a
test
The reaction AB is first order in (A). Consider the following data. Time (s) 0.0 5.0 10.0 15.0 20.0 [A] (M) 0.20 0.14 0.10 0.071 0.050 The concentration of A is Mafter 40.0 s. 1.3 e-2 O 0.17 O 3.5 e-4 0 0.025 1.2
 please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
please show work. i need to know how to do this for a
test
Question 18 1 pts The concentration of flouride ions in a saturated solution of barium flouride is _M. The Ksp of BaF is 1.7 e-6. 1.5 e-2 O 7.5 e-3 O 1.4e-4 O 3.0 e-3 3.8 e-4 1 pts Question 19
 please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass = 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k = 7.12 x 10 sec"!, what will the concentration...
please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass = 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k = 7.12 x 10 sec"!, what will the concentration...
 please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. On k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k 7.12x 10sec, what will the concentration be after 2...
please help with question 25 a and b. showing all work
25. a. (3pts) A radioactive sample of substance X has initial mass 3.25mg. It decays by a first- order reaction. If its half-life = 5.6 days, calculate its mass after 11.2 days. Given k = 0.0490. On k= b. (4pts) Compound Z decomposes by a second-order reaction. We are told the initial concentration is 0.660M. If the value of k 7.12x 10sec, what will the concentration be after 2...
 please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
please show all work. i need this for a test
Which of the following linear plots do you expect for a reaction if the kinetics are second order? (i) (ii) In[A] In[A] Time Time (iv) 1/[A] 1/[A] Time Time (v (vi) [A] [A] Time Time
 I know the final answer but I need to know all steps please show
work for good rate
Problem 1. A manufacturer has a machine that, when operational at the beginning of a day, has a probability of 0.13 of breaking down sometime during the day. When this happens, the repair is done the next day and completed at the end of that day (a) Formulate the evolution of the status of the machine as a Markov chain by identifying...
I know the final answer but I need to know all steps please show
work for good rate
Problem 1. A manufacturer has a machine that, when operational at the beginning of a day, has a probability of 0.13 of breaking down sometime during the day. When this happens, the repair is done the next day and completed at the end of that day (a) Formulate the evolution of the status of the machine as a Markov chain by identifying...
 please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0
please show work. i need this for a test
Question 21 1 pts The reaction AB is first order in A. Consider the following data: Time (s) [A] (M) 0.0 2.3 10.0 0.58 20.0 0.14 The rate constant for this reaction is s-1 0.013 O 0.030 O 3.1e-3 O 0.14 O 3.0