Homework Answers
Add Answer to:
30 2. For a Carnot heat pump cycle, a) Equal to zero b) Greater than zere...
A certain heat pump operates on a reverse Carnot cycle, heating the interior of a house...
A certain heat pump operates on a reverse Carnot cycle, heating the interior of a house to 26 degrees C, while the exterior temperature is -2 degrees C. a) What is the coefficient of performance for this heat pump? b) If the house loses 100 kJ per hour to the exterior, how much power is required to run the heat pump?
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
E A heat pump operates on a reversed Carnot cycle with a COP of 7.7. If...
 E A heat pump operates on a reversed Carnot cycle with a COP of 7.7. If it keeps a -pace at 25 C by consuming 9.6 kW of power. a. Determine the temperature of the reservoir from which the heat is absorbed, b. Determine the heating load provided by the heat pump. 6 Draw the energy flow diagram of the heat pump. d. Determine the actual COP if its irreversibility is 17 kW. e Determine the second law efficiency of...
E A heat pump operates on a reversed Carnot cycle with a COP of 7.7. If it keeps a -pace at 25 C by consuming 9.6 kW of power. a. Determine the temperature of the reservoir from which the heat is absorbed, b. Determine the heating load provided by the heat pump. 6 Draw the energy flow diagram of the heat pump. d. Determine the actual COP if its irreversibility is 17 kW. e Determine the second law efficiency of...
V6.23 Figure P6.23 shows a Carnot heat pump cycle operating at steady state with ammonia as...
 V6.23 Figure P6.23 shows a Carnot heat pump cycle operating at steady state with ammonia as the working fluid. The condenser tem perature is 49°C, with saturated vapor entering and saturated liquid exiting. The evaporator temperature -12°C a. Determine the heat transfer and work for each process, in kJ/kg of ammonia flowing. o56, 73 kJ b. Evaluate the coefficient of performance for the heat pump. 5, c. Evaluate the coefficient of performance for a Carnot refrigeration cycle operating as shown...
V6.23 Figure P6.23 shows a Carnot heat pump cycle operating at steady state with ammonia as the working fluid. The condenser tem perature is 49°C, with saturated vapor entering and saturated liquid exiting. The evaporator temperature -12°C a. Determine the heat transfer and work for each process, in kJ/kg of ammonia flowing. o56, 73 kJ b. Evaluate the coefficient of performance for the heat pump. 5, c. Evaluate the coefficient of performance for a Carnot refrigeration cycle operating as shown...
An F statistic can have what values? 1). less than or equal to zero 2). greater...
An F statistic can have what values? 1). less than or equal to zero 2). greater than or equal to zero 3). greater than zero 4). greater than or less than zero less than zero
The value of Rf is: a. greater than or equal to one b. less than or...
The value of Rf is: a. greater than or equal to one b. less than or equal to zero c. less than or equal to one d. none of the above Explain
Question 5 (10 points) Air within a piston-cylinder assembly executes a Carnot heat pump cycle. For...
 Question 5 (10 points) Air within a piston-cylinder assembly executes a Carnot heat pump cycle. For the cycle, Th = 325 C and Tc = {TC) C. The thermal energy produced by the engine has a magnitude of 200 kJ per kg of air. The pressure at the start of the isothermal expansion is 325 kPa. Determine the magnitude of the net work input, in kJ per kg of air. Your Answer: Answer
Question 5 (10 points) Air within a piston-cylinder assembly executes a Carnot heat pump cycle. For the cycle, Th = 325 C and Tc = {TC) C. The thermal energy produced by the engine has a magnitude of 200 kJ per kg of air. The pressure at the start of the isothermal expansion is 325 kPa. Determine the magnitude of the net work input, in kJ per kg of air. Your Answer: Answer
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to...
 7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
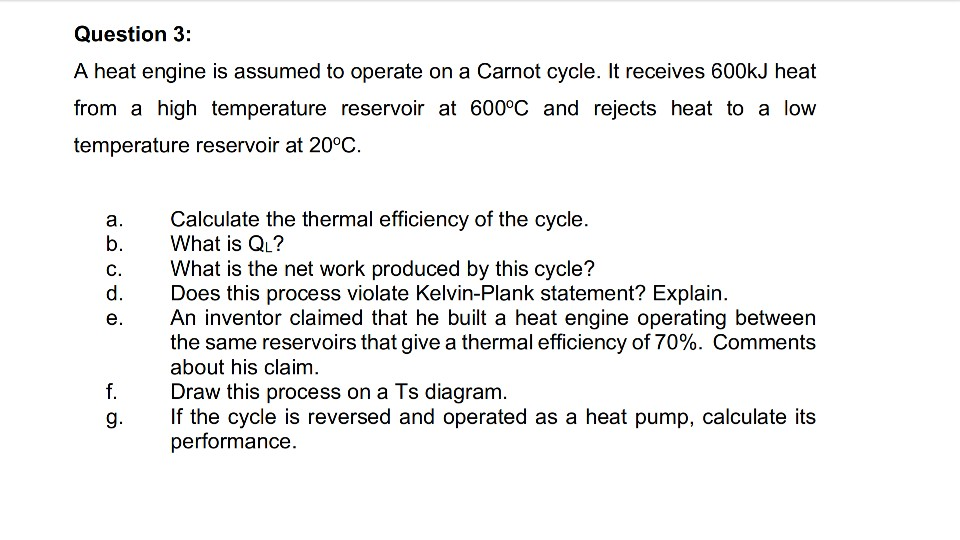 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Most questions answered within 3 hours.
-
10. Complete the table below
only using hexadecimal numbers:
AL CODE
EBX
EAX
[EAX]
mov eax,...
asked 2 minutes ago -
trust is best established through the combination of ------and
------- .
1. magnanimity and justice
2....
asked 17 minutes ago -
Blood pressure is normally taken on the upper arm at the level
of the heart. Suppose,...
asked 16 minutes ago -
Suppose that the satellite around the earth has an orbit that is
24 KM larger in...
asked 19 minutes ago -
Calculate the [OH (aq)] in limes which have a [H3O*(aq)] of 1.3 x
10 mol/L
asked 17 minutes ago -
A nozzle with a radius of 0.250 cm is attached to a garden hose
with a...
asked 28 minutes ago -
PLEASE do not use any loops for the program; only recursion is
allowed
4. Write a...
asked 37 minutes ago -
Please help me with me. I did the first part to write the operations but in...
asked 34 minutes ago -
Use Cryptool to find the Cryptographic SHA-1 hash value of the
string "abc". The calculator is...
asked 39 minutes ago -
You are attempting to calculate a firm’s free cash flow to
equity. You know the following...
asked 1 hour ago -
the following reaction occurs in a balloon containing
N2O2 gas
N2O4(g)=2NO2(g)
will the volume of the...
asked 2 hours ago -
answer the questions throughout this program
public class Day implements Comparable {
Private Boolean atWork;...
asked 2 hours ago


 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 E A heat pump operates on a reversed Carnot cycle with a COP of 7.7. If it keeps a -pace at 25 C by consuming 9.6 kW of power. a. Determine the temperature of the reservoir from which the heat is absorbed, b. Determine the heating load provided by the heat pump. 6 Draw the energy flow diagram of the heat pump. d. Determine the actual COP if its irreversibility is 17 kW. e Determine the second law efficiency of...
E A heat pump operates on a reversed Carnot cycle with a COP of 7.7. If it keeps a -pace at 25 C by consuming 9.6 kW of power. a. Determine the temperature of the reservoir from which the heat is absorbed, b. Determine the heating load provided by the heat pump. 6 Draw the energy flow diagram of the heat pump. d. Determine the actual COP if its irreversibility is 17 kW. e Determine the second law efficiency of...
 V6.23 Figure P6.23 shows a Carnot heat pump cycle operating at steady state with ammonia as the working fluid. The condenser tem perature is 49°C, with saturated vapor entering and saturated liquid exiting. The evaporator temperature -12°C a. Determine the heat transfer and work for each process, in kJ/kg of ammonia flowing. o56, 73 kJ b. Evaluate the coefficient of performance for the heat pump. 5, c. Evaluate the coefficient of performance for a Carnot refrigeration cycle operating as shown...
V6.23 Figure P6.23 shows a Carnot heat pump cycle operating at steady state with ammonia as the working fluid. The condenser tem perature is 49°C, with saturated vapor entering and saturated liquid exiting. The evaporator temperature -12°C a. Determine the heat transfer and work for each process, in kJ/kg of ammonia flowing. o56, 73 kJ b. Evaluate the coefficient of performance for the heat pump. 5, c. Evaluate the coefficient of performance for a Carnot refrigeration cycle operating as shown...
 Question 5 (10 points) Air within a piston-cylinder assembly executes a Carnot heat pump cycle. For the cycle, Th = 325 C and Tc = {TC) C. The thermal energy produced by the engine has a magnitude of 200 kJ per kg of air. The pressure at the start of the isothermal expansion is 325 kPa. Determine the magnitude of the net work input, in kJ per kg of air. Your Answer: Answer
Question 5 (10 points) Air within a piston-cylinder assembly executes a Carnot heat pump cycle. For the cycle, Th = 325 C and Tc = {TC) C. The thermal energy produced by the engine has a magnitude of 200 kJ per kg of air. The pressure at the start of the isothermal expansion is 325 kPa. Determine the magnitude of the net work input, in kJ per kg of air. Your Answer: Answer
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
 7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...
7. Consider a Carnot-cycle heat engine with water as the working fluid. The heat transfer to the water occurs at 150°C, during which process the water changes from saturated liquid to saturated vapor. The heat is rejected from the water at 25°C. (a) Show the Carnot-cycle on a T-s diagram (b) Find the properties (P, T, v, h, s) water at each state. Here v is the specific volume. (c) Determine the cycle thermal efficiency, net heat added and work...