Homework Answers


Add Answer to:
rmodynamics Spring 2018 Dr. Mazen Eldeeb 3. [Extra Credit-3% of final grade] A heat engine whose...
6000 J of heal is pul into a Carnot engine whose hoi and cold reservoirs have...
 6000 J of heal is pul into a Carnot engine whose hoi and cold reservoirs have temperatures of 550 K 320 K. Draw the direction of energy flow onto the diagram and find the heat moved into the cold reservoir, the work done by the heat engine, and the efficiency of the heat engine.
6000 J of heal is pul into a Carnot engine whose hoi and cold reservoirs have temperatures of 550 K 320 K. Draw the direction of energy flow onto the diagram and find the heat moved into the cold reservoir, the work done by the heat engine, and the efficiency of the heat engine.
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308 K. (a) If the engine receives 5113 J of heat from the reservoir at T1 in each cycle, how many joules per cycle does it deliver to the reservoir at T2? (b) How much mechanical work is performed by the engine during each cycle? (c) What is the thermal efficiency of the engine?
[3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir...
 [3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir is at 0° C. (a) Assuming this engine follows the Carnot cycle, what is the temperature of the hot reservoir? (b) Suppose the heat input to this engine was 10 J. Calculate the work done by this engine c) Suppose the heat input to this engine was 5). Calculate the heat rejected by this engine.
[3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir is at 0° C. (a) Assuming this engine follows the Carnot cycle, what is the temperature of the hot reservoir? (b) Suppose the heat input to this engine was 10 J. Calculate the work done by this engine c) Suppose the heat input to this engine was 5). Calculate the heat rejected by this engine.
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
For a Carnot engine with 10 moles of ideal gas (Cv = 1.5 nR) and operating...
For a Carnot engine with 10 moles of ideal gas (Cv = 1.5 nR) and operating between a hot reservoir of 500 K and a cold reservoir of 300 K, a) What would be the heat exchanges (q1) and entropy change (∆S1) for step 1, where the gas reversibly and isothermally expands to double its volume (V2 = 2 V1) at 500 K? b) What would be the heat exchanges (q3) and entropy change (∆S3) for step 3, where the...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
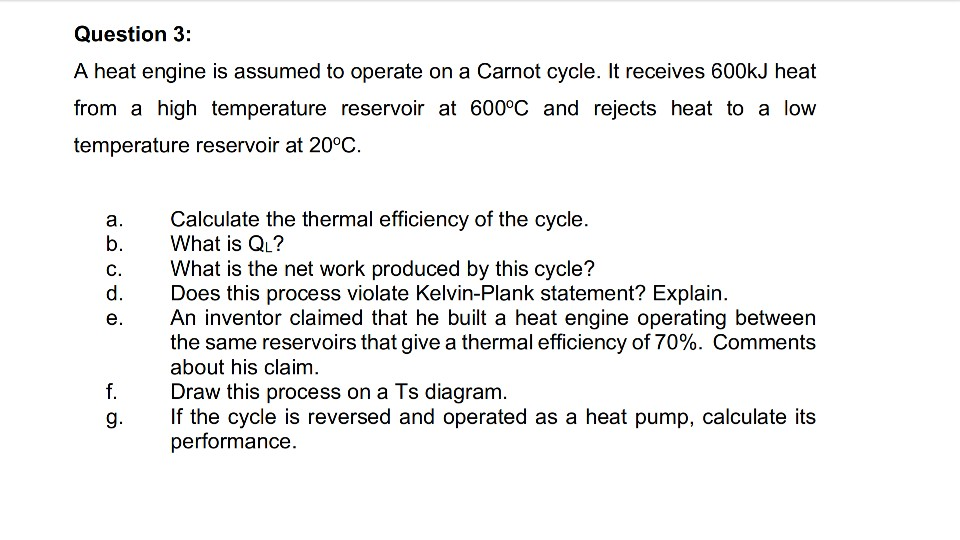 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A Carnot engine works between two heat reservoirs at temperatures Th = 360 K and Tc...
A Carnot engine works between two heat reservoirs at temperatures Th = 360 K and Tc = 240 K. (a) What is its efficiency? % (b) If it absorbs 95 J from the hot reservoir during each cycle, how much work does it do? J (c) How much heat does it give off during each cycle? J (d) What is the COP of this engine when it works as a refrigerator between the same two reservoirs?
For a Carnot engine with 10 moles of ideal gas (Cv= 1.5 nR) and operatingbetween a...
For a Carnot engine with 10 moles of ideal gas (Cv= 1.5 nR) and operatingbetween a hot reservoir of 500 K and a cold reservoir of 300 K,a. (6 Points) What would be the heat exchanges (q1) and entropy change (∆S1) for step 1, where thegas reversibly and isothermally expands to double its volume (V2= 2 V1) at 500 K?b. (6 Points) What would be the heat exchanges (q3) and entropy change (∆S3) for step 3, where thegas is reversibly...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of...
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
Most questions answered within 3 hours.
-
Assume Kw = 1.01 ✕ 10−14
For pure water, we can calculate [H3O+ ] = [OH...
asked 22 minutes ago -
Suppose that on a temperature scale X, water boils at 203.0°X
and freezes at -105.7°X. What...
asked 1 hour ago -
BaS crystallizes in a cubic unit cell with S2- ions on each
corner and each face....
asked 1 hour ago -
A. 0≤P(Oi)≤10≤P(Oi)≤1 for each i
B. P(Oi)≤0P(Oi)≤0
C. P(Oi)=1+P(OCi)P(Oi)=1+P(OiC)
D. P(Oi)≥1P(Oi)≥1
If an experiment consists of...
asked 3 hours ago -
A battery has an emf of 9.20V and an internal resistance of 1.20
ohm. a)What resistance...
asked 3 hours ago -
The area of an elastic circular loop decreases at a constant
rate, dA/dt = −6.60×10−3 m2/s...
asked 4 hours ago -
The denaturation of proteins can be described by the
equilibrium
F⇌U
where F and U represent...
asked 5 hours ago -
Please answer what the maximum and minimum force is, and the
angle on the ion is...
asked 5 hours ago -
implement a program that reads a number of rows and a symbol.
The program will draw...
asked 5 hours ago -
Assume that when adults with smartphones are randomly selected,
45% use them in meetings or classes....
asked 6 hours ago -
Determine the number of formula units of
Na2SO4 and moles of oxygen contained in 8.11
moles...
asked 6 hours ago -
Explain in steps on the following code
What would be the output when executed
using System;...
asked 6 hours ago
![rmodynamics Spring 2018 Dr. Mazen Eldeeb 3. [Extra Credit-3% of final grade] A heat engine whose thermal efficiency is 60% uesa hot reservoir at 1300 K and a cold reservoir at 500 K. Calculate the entropy chang nine the two reservoirs when 1 Btu of heat is transferre d from the hot reservoir to principle? If the thermal efi the engine. tropy efficiency of the heat engine is 65%, will the in Carnot efficiency of this engine? Hint: 1 Btu e increase in entropy principle still be satisfied? What is the 1](http://img.homeworklib.com/questions/a90e9c20-390c-11ec-bb94-8762b45810f5.png?x-oss-process=image/resize,w_560)
 6000 J of heal is pul into a Carnot engine whose hoi and cold reservoirs have temperatures of 550 K 320 K. Draw the direction of energy flow onto the diagram and find the heat moved into the cold reservoir, the work done by the heat engine, and the efficiency of the heat engine.
6000 J of heal is pul into a Carnot engine whose hoi and cold reservoirs have temperatures of 550 K 320 K. Draw the direction of energy flow onto the diagram and find the heat moved into the cold reservoir, the work done by the heat engine, and the efficiency of the heat engine.
 [3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir is at 0° C. (a) Assuming this engine follows the Carnot cycle, what is the temperature of the hot reservoir? (b) Suppose the heat input to this engine was 10 J. Calculate the work done by this engine c) Suppose the heat input to this engine was 5). Calculate the heat rejected by this engine.
[3] A heat engine is reported to operate with 25 % efficiency when the cold reservoir is at 0° C. (a) Assuming this engine follows the Carnot cycle, what is the temperature of the hot reservoir? (b) Suppose the heat input to this engine was 10 J. Calculate the work done by this engine c) Suppose the heat input to this engine was 5). Calculate the heat rejected by this engine.
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...