Homework Answers



Thank you.
Add Answer to:
2. Two reversible Carnot heat engines operate in series between a source at 427°C and a...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
thermo 2) The work output of a Carnot heat engine is 900 kJ and it rejects...
 thermo
2) The work output of a Carnot heat engine is 900 kJ and it rejects 250 kJ of heat to the heat sink at 24°C. Determine: a. The temperature of the source b. The heat supplied to the heat engine. IH QH HE 900 kJ 150 kJ 27 C
thermo
2) The work output of a Carnot heat engine is 900 kJ and it rejects 250 kJ of heat to the heat sink at 24°C. Determine: a. The temperature of the source b. The heat supplied to the heat engine. IH QH HE 900 kJ 150 kJ 27 C
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive...
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive a Carnot refrigerator that removes heat from -15oC space at a rate of 400kJ/min and rejects the heat to 25 oC environment. (a) Find the rate of heat supplied to the heat engine (b) Find the total rate of heat rejection to the environment
pls i need this step by step pls 2. Two Carnot engines are operated in series...
 pls i need this step by step pls
2. Two Carnot engines are operated in series with the exhaust (heat output) of the first engine being the input of the second engine. The upper temperature of this combination is 260F, the lower temperature is 40F. If each engine has the same thermal efficiency, determine the exhaust temperature of the first engine (the inlet temperature of the second engine). Ans: T = 140F
pls i need this step by step pls
2. Two Carnot engines are operated in series with the exhaust (heat output) of the first engine being the input of the second engine. The upper temperature of this combination is 260F, the lower temperature is 40F. If each engine has the same thermal efficiency, determine the exhaust temperature of the first engine (the inlet temperature of the second engine). Ans: T = 140F
Fall 2019 PChem 2. A certain heat engine operates between 590 °C and 170 °C. (a)...
 Fall 2019 PChem 2. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
Fall 2019 PChem 2. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
Operating in series are two reversible heat pumps. Heat transfer gives energy to the first cycle...
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Fall 2019 PChem 7. A certain heat engine operates between 590 °C and 170 °C. (a)...
 Fall 2019 PChem 7. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
Fall 2019 PChem 7. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive...
A Carnot heat engine works between 450oC and 25oC. The work output is used to drive a Carnot refrigerator that removes heat from -15oC space at a rate of 400kJ/min and rejects the heat to 25 oC environment. Find the rate of heat supplied to the heat engine Find the total rate of heat rejection to the environment ii) In the steady state of a steam turbine operation, steam enters a turbine at a rate of 25000 kg/hr at 4MPa...
thermodynamics ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It...
 thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
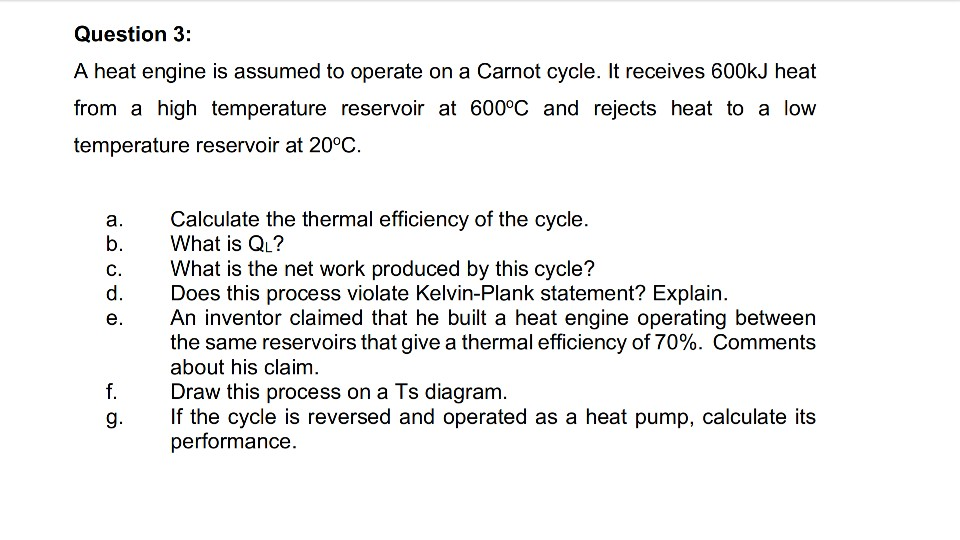 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
Most questions answered within 3 hours.
-
You are attempting to calculate a firm’s free cash flow to
equity. You know the following...
asked 18 minutes ago -
the following reaction occurs in a balloon containing
N2O2 gas
N2O4(g)=2NO2(g)
will the volume of the...
asked 53 minutes ago -
answer the questions throughout this program
public class Day implements Comparable {
Private Boolean atWork;...
asked 1 hour ago -
This is C++ code for parking fee management program
#include <iostream>
#include <iomanip>
using namespace std;...
asked 1 hour ago -
The free energy change for the following reaction at 25 °C, when
[Sn2+] = 1.17 M...
asked 2 hours ago -
An MNE is this kind of industry when competition in one country
is essentially independent of...
asked 4 hours ago -
. For this set of questions, determine what
proportion of a normal distribution is located betweeneach...
asked 5 hours ago -
A college student is employed as a door-to-door newspaper
salesman. Historical data suggests that the student...
asked 5 hours ago -
MATLAB HW 11 problem using Switch Case and Input commands
Write a script file that calculates...
asked 5 hours ago -
Considering gravitational time dilation, calculate the time that
passes in Earth’s surface while 1 hour passes...
asked 6 hours ago -
Minitab Problem: Take the Lake Hume June rainfall data and find
use the processes outlined in...
asked 7 hours ago -
X Company is trying to decide whether to continue using old
equipment to make Product A...
asked 7 hours ago

 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
 thermo
2) The work output of a Carnot heat engine is 900 kJ and it rejects 250 kJ of heat to the heat sink at 24°C. Determine: a. The temperature of the source b. The heat supplied to the heat engine. IH QH HE 900 kJ 150 kJ 27 C
thermo
2) The work output of a Carnot heat engine is 900 kJ and it rejects 250 kJ of heat to the heat sink at 24°C. Determine: a. The temperature of the source b. The heat supplied to the heat engine. IH QH HE 900 kJ 150 kJ 27 C
 pls i need this step by step pls
2. Two Carnot engines are operated in series with the exhaust (heat output) of the first engine being the input of the second engine. The upper temperature of this combination is 260F, the lower temperature is 40F. If each engine has the same thermal efficiency, determine the exhaust temperature of the first engine (the inlet temperature of the second engine). Ans: T = 140F
pls i need this step by step pls
2. Two Carnot engines are operated in series with the exhaust (heat output) of the first engine being the input of the second engine. The upper temperature of this combination is 260F, the lower temperature is 40F. If each engine has the same thermal efficiency, determine the exhaust temperature of the first engine (the inlet temperature of the second engine). Ans: T = 140F
 Fall 2019 PChem 2. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
Fall 2019 PChem 2. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
 Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
Operating in series are two reversible heat pumps. Heat transfer
gives energy to the first cycle from a cold reservoir at 105 K and
rejects energy by heat transfer to a reservoir at an intermediate
temperature T greater than 105 K. The second cycle receives energy
by heat transfer from the reservoir at T and rejects energy by heat
transfer to a higher-temperature reservoir at 1200 K. If the heat
pump cycles have the same co-efficient of performance, calculate:
Low...
 Fall 2019 PChem 7. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
Fall 2019 PChem 7. A certain heat engine operates between 590 °C and 170 °C. (a) What is the maximum efficiency of the engine? (b) How much heat is needed from the hot source for each 1.0 kJ of maximum work done? (c) For each 1.0 kJ of heat discharged into the cold sink, how much heat is supplied by the hot source? How much heat is discharged into the cold sink in a reversible process for each 1.0 kJ...
 thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...