Two different gases occupy two separate bulbs. (Figure 1) Consider the process that occurs when the stopcock separati...
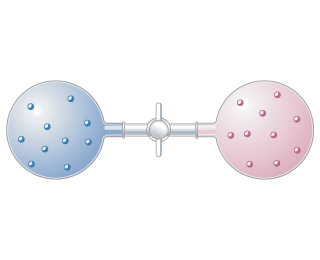
Two different gases occupy two separate bulbs. (Figure 1) Consider the process that occurs when the stopcock separating the gases is opened, assuming the gases behave ideally.
Part A
Predict the sign of ΔH for the process.
Predict the sign of for the process.
| ΔH > 0 | |
| ΔH < 0 | |
| ΔH = 0 |
Part B
Predict the sign of ΔS for the process.
Predict the sign of for the process.
| ΔS > 0 | |
| ΔS < 0 | |
| ΔS = 0 |
Part C
Is the process that occurs when the stopcock is opened a reversible one?
Is the process that occurs when the stopcock is opened a reversible one?
| yes | |
| no |
Part D
How does the process affect the enthalpy of the surroundings?
How does the process affect the enthalpy of the surroundings?
| ΔHsurr > 0 | |
| ΔHsurr < 0 | |
| ΔHsurr = 0 |
Homework Answers
ΔHmix = 0. Because the enthalpy of mixing for two ideal gasses is 0.
ΔSmix =-nR (x1 ln x1 + x2 ln x2). In this expression x1 and x2 are positive numbers lying between 0 &1. Thus ln x1 and ln x2 must be negative numbers. Therefore entropy change in this process is positive. So, ΔS > 0.
The Gibb's free energy change of mixing is calculated as ΔGmix = ΔHmix -TΔSmix.
Now, ΔHmix = 0 and ΔSmix > 0. Thus, ΔGmix < 0 for all temperature. As the process is spontaneous the mixing can not be reversible.
As the enthalpy change of the system is 0 that of the surrounding is also 0. So, ΔHsurr = 0
Add Answer to:
Two different gases occupy two separate bulbs. (Figure 1) Consider the process that occurs when the stopcock separati...
Consider the apparatus shown in the drawing O2 2.0 L 1.0 atm 25°C 3.0 L 2.0 atm 25℃ Part A When the valve between the two containers is opened and the gases allowed to mix, how does the volume occupi...
 Consider the apparatus shown in the drawing O2 2.0 L 1.0 atm 25°C 3.0 L 2.0 atm 25℃ Part A When the valve between the two containers is opened and the gases allowed to mix, how does the volume occupied by the N2 gas change? Express your answer using two significant figures. ANSWER of 17 215/2018, 8:25 A com myct assignmentPrint View?disp. CH 10 HW Part B What is the partial pressure of N2 after mixing? Expre ANSWER ss your...
Consider the apparatus shown in the drawing O2 2.0 L 1.0 atm 25°C 3.0 L 2.0 atm 25℃ Part A When the valve between the two containers is opened and the gases allowed to mix, how does the volume occupied by the N2 gas change? Express your answer using two significant figures. ANSWER of 17 215/2018, 8:25 A com myct assignmentPrint View?disp. CH 10 HW Part B What is the partial pressure of N2 after mixing? Expre ANSWER ss your...
Help on these would be great! 111) Th of 1 mol of A to B absorbs...
 Help on these would be great!
111) Th of 1 mol of A to B absorbs 15 kcal of heat from the surroundings when it occurs irreversibly. doing no work. At the same T and P, reversible conversion of 1 mol of B to A does 10 kcal of for work done on system, heat absorbed by system.) a) i) For A → B, determine qirrev and wirrev ermodynamics of a Cyclic Process: At 293 K and 1 atm pressure,...
Help on these would be great!
111) Th of 1 mol of A to B absorbs 15 kcal of heat from the surroundings when it occurs irreversibly. doing no work. At the same T and P, reversible conversion of 1 mol of B to A does 10 kcal of for work done on system, heat absorbed by system.) a) i) For A → B, determine qirrev and wirrev ermodynamics of a Cyclic Process: At 293 K and 1 atm pressure,...
1,4? Part I: Choose five of these questions. (12 points each) 1. Here is the van...
 1,4?
Part I: Choose five of these questions. (12 points each) 1. Here is the van der Waals Equation for one mole of gas P Given this equation, how does the infinitesimal change in pressure, or as specific as possible. (In other words, evaluate the derivatives.) with d V and dT? B e-NV, where is the sity her of molecules and V is the fshe wall perpendicular to the 2. A sample of gas molecules of density N e e...
1,4?
Part I: Choose five of these questions. (12 points each) 1. Here is the van der Waals Equation for one mole of gas P Given this equation, how does the infinitesimal change in pressure, or as specific as possible. (In other words, evaluate the derivatives.) with d V and dT? B e-NV, where is the sity her of molecules and V is the fshe wall perpendicular to the 2. A sample of gas molecules of density N e e...
number 9 please MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answen...
 number 9 please
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answen the sers the question. 6) A particular 12V battery is bas battery is based on a reaction having a standard cell potential, E 1.92 V. What happens when the battery "dies? A) E° - 12 V and E = 12 V B) E = +1.88 V and E= 12 V C) E = +1.88 V and E=OV D) E-12 V and E-OV ) 1)...
number 9 please
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answen the sers the question. 6) A particular 12V battery is bas battery is based on a reaction having a standard cell potential, E 1.92 V. What happens when the battery "dies? A) E° - 12 V and E = 12 V B) E = +1.88 V and E= 12 V C) E = +1.88 V and E=OV D) E-12 V and E-OV ) 1)...
3,9? Part I: Choose five of these questions. (12 points each) Ana 1. Here is the...
 3,9?
Part I: Choose five of these questions. (12 points each) Ana 1. Here is the van der Waals Equation for one mole of gas: P -by vary with dV and dT? Bethe Given this equation, how does the infinitesimal change in pressure, dl. as specific as possible. (In other words, evaluate the derivatives.) andard 2. A sample of gas molecules of density D N / Vwhere N is the numbe volume) is moving with a speed y, in the...
3,9?
Part I: Choose five of these questions. (12 points each) Ana 1. Here is the van der Waals Equation for one mole of gas: P -by vary with dV and dT? Bethe Given this equation, how does the infinitesimal change in pressure, dl. as specific as possible. (In other words, evaluate the derivatives.) andard 2. A sample of gas molecules of density D N / Vwhere N is the numbe volume) is moving with a speed y, in the...
REPORT BOOK QUESTIONS These questions from the Report Book are included in the manual to help you prepare for the E...
 REPORT BOOK QUESTIONS These questions from the Report Book are included in the manual to help you prepare for the Experiment and the Computer Preliminary Exercises. a) Write an equation describing the reaction of HCI with water. b) Hence, write an equation describing the reaction between an aqueous solution of ammonia and hydrochloric acid. Indicate the acid base conjugate pairs. Calculate the energy absorbed by the calorimeter alone, calorimeter Refer to Laboratory Manual, Page Calculate the energy absorbed by the...
REPORT BOOK QUESTIONS These questions from the Report Book are included in the manual to help you prepare for the Experiment and the Computer Preliminary Exercises. a) Write an equation describing the reaction of HCI with water. b) Hence, write an equation describing the reaction between an aqueous solution of ammonia and hydrochloric acid. Indicate the acid base conjugate pairs. Calculate the energy absorbed by the calorimeter alone, calorimeter Refer to Laboratory Manual, Page Calculate the energy absorbed by the...
The first two pages are a background of the lab and there is a page with...
 The first two pages are a background of the lab and there is a
page with the results. I would appreciate any help with this. If
needed I have the pre-lab questions as well. I added in the pre-lab
questions I will get them answered and updated again if that is
what is needed.
Report Sheet Name Pre-Lab Questions [Ni(H0) ] + + 6 NHỮ[Ni(NH] + + H2O 1. Given the spontaneous chemical reaction shown above and the fact that...
The first two pages are a background of the lab and there is a
page with the results. I would appreciate any help with this. If
needed I have the pre-lab questions as well. I added in the pre-lab
questions I will get them answered and updated again if that is
what is needed.
Report Sheet Name Pre-Lab Questions [Ni(H0) ] + + 6 NHỮ[Ni(NH] + + H2O 1. Given the spontaneous chemical reaction shown above and the fact that...
The first two pages are the background of the lab. I would appreciate any help with...
 The first two pages are the background of the lab. I would
appreciate any help with the pre-lab questions, even if you cannot
answer them all, so I could continue to the post-lab on my own.
Experiment Thermodynamics: Entropy vs. Enthalpy OH, Background The formation of a Lewis Acid-Base complex occurs when you have a lone pair of electrons being donated to an electropositive ion or atom. Common examples are the Lewis Acid-Base complexes that most metal ions form in...
The first two pages are the background of the lab. I would
appreciate any help with the pre-lab questions, even if you cannot
answer them all, so I could continue to the post-lab on my own.
Experiment Thermodynamics: Entropy vs. Enthalpy OH, Background The formation of a Lewis Acid-Base complex occurs when you have a lone pair of electrons being donated to an electropositive ion or atom. Common examples are the Lewis Acid-Base complexes that most metal ions form in...
Unit 3 Study Resource Meiosis • Process by which diploid cells create haploid cells NOT part...
 Unit 3 Study Resource Meiosis • Process by which diploid cells create haploid cells NOT part of the cell cycle > only some cells ever undergo meiosis During meiosis I, homologous chromosomes line up to allow them to be separated into two new cells o They can become "tangled" during this phase, which leads to crossing-over (rearranging the alleles) O Result of meiosis I is two non-identical haploid cells Meiosis Il looks very similar to mitosis, in that sister chromatids...
Unit 3 Study Resource Meiosis • Process by which diploid cells create haploid cells NOT part of the cell cycle > only some cells ever undergo meiosis During meiosis I, homologous chromosomes line up to allow them to be separated into two new cells o They can become "tangled" during this phase, which leads to crossing-over (rearranging the alleles) O Result of meiosis I is two non-identical haploid cells Meiosis Il looks very similar to mitosis, in that sister chromatids...
Multiple-Choice Questions (worth two points each) 1. Which of the following describes the process in which...
 Multiple-Choice Questions (worth two points each) 1. Which of the following describes the process in which one adopts patterns of behavior that lead to greater life satisfaction? A. wellness B. health C. social determination D. self-efficacy 2. The Stages of Change Model of health behavior change emphasizes that A. change happens as a process. B. people change only when faced with an illness. C. change occurs only when the environment supports it. D. changes are more effective when based on...
Multiple-Choice Questions (worth two points each) 1. Which of the following describes the process in which one adopts patterns of behavior that lead to greater life satisfaction? A. wellness B. health C. social determination D. self-efficacy 2. The Stages of Change Model of health behavior change emphasizes that A. change happens as a process. B. people change only when faced with an illness. C. change occurs only when the environment supports it. D. changes are more effective when based on...
Most questions answered within 3 hours.
-
Assume Kw = 1.01 ✕ 10−14
For pure water, we can calculate [H3O+ ] = [OH...
asked 1 minute ago -
Suppose that on a temperature scale X, water boils at 203.0°X
and freezes at -105.7°X. What...
asked 50 minutes ago -
BaS crystallizes in a cubic unit cell with S2- ions on each
corner and each face....
asked 1 hour ago -
A. 0≤P(Oi)≤10≤P(Oi)≤1 for each i
B. P(Oi)≤0P(Oi)≤0
C. P(Oi)=1+P(OCi)P(Oi)=1+P(OiC)
D. P(Oi)≥1P(Oi)≥1
If an experiment consists of...
asked 2 hours ago -
A battery has an emf of 9.20V and an internal resistance of 1.20
ohm. a)What resistance...
asked 2 hours ago -
The area of an elastic circular loop decreases at a constant
rate, dA/dt = −6.60×10−3 m2/s...
asked 3 hours ago -
The denaturation of proteins can be described by the
equilibrium
F⇌U
where F and U represent...
asked 5 hours ago -
Please answer what the maximum and minimum force is, and the
angle on the ion is...
asked 5 hours ago -
implement a program that reads a number of rows and a symbol.
The program will draw...
asked 5 hours ago -
Assume that when adults with smartphones are randomly selected,
45% use them in meetings or classes....
asked 5 hours ago -
Determine the number of formula units of
Na2SO4 and moles of oxygen contained in 8.11
moles...
asked 5 hours ago -
Explain in steps on the following code
What would be the output when executed
using System;...
asked 5 hours ago
 Consider the apparatus shown in the drawing O2 2.0 L 1.0 atm 25°C 3.0 L 2.0 atm 25℃ Part A When the valve between the two containers is opened and the gases allowed to mix, how does the volume occupied by the N2 gas change? Express your answer using two significant figures. ANSWER of 17 215/2018, 8:25 A com myct assignmentPrint View?disp. CH 10 HW Part B What is the partial pressure of N2 after mixing? Expre ANSWER ss your...
Consider the apparatus shown in the drawing O2 2.0 L 1.0 atm 25°C 3.0 L 2.0 atm 25℃ Part A When the valve between the two containers is opened and the gases allowed to mix, how does the volume occupied by the N2 gas change? Express your answer using two significant figures. ANSWER of 17 215/2018, 8:25 A com myct assignmentPrint View?disp. CH 10 HW Part B What is the partial pressure of N2 after mixing? Expre ANSWER ss your...
 Help on these would be great!
111) Th of 1 mol of A to B absorbs 15 kcal of heat from the surroundings when it occurs irreversibly. doing no work. At the same T and P, reversible conversion of 1 mol of B to A does 10 kcal of for work done on system, heat absorbed by system.) a) i) For A → B, determine qirrev and wirrev ermodynamics of a Cyclic Process: At 293 K and 1 atm pressure,...
Help on these would be great!
111) Th of 1 mol of A to B absorbs 15 kcal of heat from the surroundings when it occurs irreversibly. doing no work. At the same T and P, reversible conversion of 1 mol of B to A does 10 kcal of for work done on system, heat absorbed by system.) a) i) For A → B, determine qirrev and wirrev ermodynamics of a Cyclic Process: At 293 K and 1 atm pressure,...
 1,4?
Part I: Choose five of these questions. (12 points each) 1. Here is the van der Waals Equation for one mole of gas P Given this equation, how does the infinitesimal change in pressure, or as specific as possible. (In other words, evaluate the derivatives.) with d V and dT? B e-NV, where is the sity her of molecules and V is the fshe wall perpendicular to the 2. A sample of gas molecules of density N e e...
1,4?
Part I: Choose five of these questions. (12 points each) 1. Here is the van der Waals Equation for one mole of gas P Given this equation, how does the infinitesimal change in pressure, or as specific as possible. (In other words, evaluate the derivatives.) with d V and dT? B e-NV, where is the sity her of molecules and V is the fshe wall perpendicular to the 2. A sample of gas molecules of density N e e...
 number 9 please
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answen the sers the question. 6) A particular 12V battery is bas battery is based on a reaction having a standard cell potential, E 1.92 V. What happens when the battery "dies? A) E° - 12 V and E = 12 V B) E = +1.88 V and E= 12 V C) E = +1.88 V and E=OV D) E-12 V and E-OV ) 1)...
number 9 please
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answen the sers the question. 6) A particular 12V battery is bas battery is based on a reaction having a standard cell potential, E 1.92 V. What happens when the battery "dies? A) E° - 12 V and E = 12 V B) E = +1.88 V and E= 12 V C) E = +1.88 V and E=OV D) E-12 V and E-OV ) 1)...
 3,9?
Part I: Choose five of these questions. (12 points each) Ana 1. Here is the van der Waals Equation for one mole of gas: P -by vary with dV and dT? Bethe Given this equation, how does the infinitesimal change in pressure, dl. as specific as possible. (In other words, evaluate the derivatives.) andard 2. A sample of gas molecules of density D N / Vwhere N is the numbe volume) is moving with a speed y, in the...
3,9?
Part I: Choose five of these questions. (12 points each) Ana 1. Here is the van der Waals Equation for one mole of gas: P -by vary with dV and dT? Bethe Given this equation, how does the infinitesimal change in pressure, dl. as specific as possible. (In other words, evaluate the derivatives.) andard 2. A sample of gas molecules of density D N / Vwhere N is the numbe volume) is moving with a speed y, in the...
 REPORT BOOK QUESTIONS These questions from the Report Book are included in the manual to help you prepare for the Experiment and the Computer Preliminary Exercises. a) Write an equation describing the reaction of HCI with water. b) Hence, write an equation describing the reaction between an aqueous solution of ammonia and hydrochloric acid. Indicate the acid base conjugate pairs. Calculate the energy absorbed by the calorimeter alone, calorimeter Refer to Laboratory Manual, Page Calculate the energy absorbed by the...
REPORT BOOK QUESTIONS These questions from the Report Book are included in the manual to help you prepare for the Experiment and the Computer Preliminary Exercises. a) Write an equation describing the reaction of HCI with water. b) Hence, write an equation describing the reaction between an aqueous solution of ammonia and hydrochloric acid. Indicate the acid base conjugate pairs. Calculate the energy absorbed by the calorimeter alone, calorimeter Refer to Laboratory Manual, Page Calculate the energy absorbed by the...
 The first two pages are a background of the lab and there is a
page with the results. I would appreciate any help with this. If
needed I have the pre-lab questions as well. I added in the pre-lab
questions I will get them answered and updated again if that is
what is needed.
Report Sheet Name Pre-Lab Questions [Ni(H0) ] + + 6 NHỮ[Ni(NH] + + H2O 1. Given the spontaneous chemical reaction shown above and the fact that...
The first two pages are a background of the lab and there is a
page with the results. I would appreciate any help with this. If
needed I have the pre-lab questions as well. I added in the pre-lab
questions I will get them answered and updated again if that is
what is needed.
Report Sheet Name Pre-Lab Questions [Ni(H0) ] + + 6 NHỮ[Ni(NH] + + H2O 1. Given the spontaneous chemical reaction shown above and the fact that...
 The first two pages are the background of the lab. I would
appreciate any help with the pre-lab questions, even if you cannot
answer them all, so I could continue to the post-lab on my own.
Experiment Thermodynamics: Entropy vs. Enthalpy OH, Background The formation of a Lewis Acid-Base complex occurs when you have a lone pair of electrons being donated to an electropositive ion or atom. Common examples are the Lewis Acid-Base complexes that most metal ions form in...
The first two pages are the background of the lab. I would
appreciate any help with the pre-lab questions, even if you cannot
answer them all, so I could continue to the post-lab on my own.
Experiment Thermodynamics: Entropy vs. Enthalpy OH, Background The formation of a Lewis Acid-Base complex occurs when you have a lone pair of electrons being donated to an electropositive ion or atom. Common examples are the Lewis Acid-Base complexes that most metal ions form in...
 Unit 3 Study Resource Meiosis • Process by which diploid cells create haploid cells NOT part of the cell cycle > only some cells ever undergo meiosis During meiosis I, homologous chromosomes line up to allow them to be separated into two new cells o They can become "tangled" during this phase, which leads to crossing-over (rearranging the alleles) O Result of meiosis I is two non-identical haploid cells Meiosis Il looks very similar to mitosis, in that sister chromatids...
Unit 3 Study Resource Meiosis • Process by which diploid cells create haploid cells NOT part of the cell cycle > only some cells ever undergo meiosis During meiosis I, homologous chromosomes line up to allow them to be separated into two new cells o They can become "tangled" during this phase, which leads to crossing-over (rearranging the alleles) O Result of meiosis I is two non-identical haploid cells Meiosis Il looks very similar to mitosis, in that sister chromatids...
 Multiple-Choice Questions (worth two points each) 1. Which of the following describes the process in which one adopts patterns of behavior that lead to greater life satisfaction? A. wellness B. health C. social determination D. self-efficacy 2. The Stages of Change Model of health behavior change emphasizes that A. change happens as a process. B. people change only when faced with an illness. C. change occurs only when the environment supports it. D. changes are more effective when based on...
Multiple-Choice Questions (worth two points each) 1. Which of the following describes the process in which one adopts patterns of behavior that lead to greater life satisfaction? A. wellness B. health C. social determination D. self-efficacy 2. The Stages of Change Model of health behavior change emphasizes that A. change happens as a process. B. people change only when faced with an illness. C. change occurs only when the environment supports it. D. changes are more effective when based on...