Which of the following statements are true concerning NO2-? true false There are two resonance structures for...
Which of the following statements are true concerning NO2-?
true false There are two resonance structures for
NO2-.
true false One of the nitrogen-oxygen bonds is longer
than the others.
true false There are two sigma bonds in
NO2-.
true false The central nitrogen atom is sp2
hybridized.
true false The bond angles about the central nitrogen
atom are about 120o.
Homework Answers

true There are two resonance structures for NO2-.
false One of the nitrogen-oxygen bonds is longer than the others.
true There are two sigma bonds in NO2-.
true The central nitrogen atom is sp2 hybridized.
true The bond angles about the central nitrogen atom are about 120o.
The two most important resonance structures are shown below.
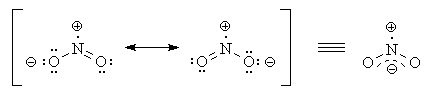
trigonal-planar configuration, with 120
Add Answer to:
Which of the following statements are true concerning NO2-? true false There are two resonance structures for...
Which of the following statements are true concerning CO32? 2-2 All carbon-oxygen bonds are equal in...
 Which of the following statements are true concerning CO32? 2-2 All carbon-oxygen bonds are equal in bond length. There are two sigma bonds in CO32 The bond angles about the central carbon atom are about 109°. There are two resonance structures for CO32 The central carbon atom is sp2 hybridized. true
Which of the following statements are true concerning CO32? 2-2 All carbon-oxygen bonds are equal in bond length. There are two sigma bonds in CO32 The bond angles about the central carbon atom are about 109°. There are two resonance structures for CO32 The central carbon atom is sp2 hybridized. true
Determine if each of the statements is True or False about the resonance hybrid of PO43−....
Determine if each of the statements is True or False about the resonance hybrid of PO43−. 1) The central atom, P, has one lone pair electrons. 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. 4) All four resonance structures of PO43− contribute equally to...
Determine if each of the statements is True or False about the resonance hybrid of PO4-....
 Determine if each of the statements is True or False about the resonance hybrid of PO4-. 1) The central atom, P, has one lone pair electrons. (Select] 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43-. [Select] 5)...
Determine if each of the statements is True or False about the resonance hybrid of PO4-. 1) The central atom, P, has one lone pair electrons. (Select] 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43-. [Select] 5)...
Question 10 1 pts Determine if each of the statements is True or False about the...
 Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of NO3 1) There are three equivalent resonance structures of NO3. Select 1 2) All three NO (nitrogen oxygen) bonds are equivalent in the resonance hybrid. I Select 1 3) The resonance hybrid violates the octet rule. Select 1 4) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. Select 1 5) There are two single...
Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of NO3 1) There are three equivalent resonance structures of NO3. Select 1 2) All three NO (nitrogen oxygen) bonds are equivalent in the resonance hybrid. I Select 1 3) The resonance hybrid violates the octet rule. Select 1 4) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. Select 1 5) There are two single...
Determine if each of the statements is True or False about the resonance hybrid of PO43- [Select] 1) The central at...
 Determine if each of the statements is True or False about the resonance hybrid of PO43- [Select] 1) The central atom, P, has one lone pair electrons. 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. [Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. [Select] 4) All four resonance structures of PO43 contribute equally to the resonance hybrid of PO3- [Select ]...
Determine if each of the statements is True or False about the resonance hybrid of PO43- [Select] 1) The central atom, P, has one lone pair electrons. 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. [Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. [Select] 4) All four resonance structures of PO43 contribute equally to the resonance hybrid of PO3- [Select ]...
Question 10 1 pts Determine if each of the statements is True or False about the...
 Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of PO4 1) The central atom, P, has one lone pair electrons. True 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. True 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. True 4) All four resonance structures of PO43- contribute equally to the resonance hybrid...
Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of PO4 1) The central atom, P, has one lone pair electrons. True 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. True 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. True 4) All four resonance structures of PO43- contribute equally to the resonance hybrid...
Determine if each of the statements is True or False about the resonance hybrid of PO43- 1) The central atom, P, has on...
 Determine if each of the statements is True or False about the resonance hybrid of PO43- 1) The central atom, P, has one lone pair electrons. [Select) 2) There are three single bonds and one double bond around the Patom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43- [Select] 5) The...
Determine if each of the statements is True or False about the resonance hybrid of PO43- 1) The central atom, P, has one lone pair electrons. [Select) 2) There are three single bonds and one double bond around the Patom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43- [Select] 5) The...
Consider the resonance structures of formate. 0 Select the true statements about the resonance structures. Each...
 Consider the resonance structures of formate. 0 Select the true statements about the resonance structures. Each oxygen atom has a double bond 50% of the time. The actual structure of formate is an average of the two resonance forms. The actual structure of formate switches back and forth between the two resonance forms. Each carbon-oxygen bond is somewhere between a single and double bond.
Consider the resonance structures of formate. 0 Select the true statements about the resonance structures. Each oxygen atom has a double bond 50% of the time. The actual structure of formate is an average of the two resonance forms. The actual structure of formate switches back and forth between the two resonance forms. Each carbon-oxygen bond is somewhere between a single and double bond.
1 pts Question 10 Determine if each of the statements is True or False about the resonance hybrid of C032. 1) All t...
 1 pts Question 10 Determine if each of the statements is True or False about the resonance hybrid of C032. 1) All three resonance structures of CO3 contribute equally to the resonance hybrid of CO2. [Select ] 2) The central atom, C, has as a formal charge of -2 in the resonance hybrid. Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select) Select 4) The resonance hybrid follows the octet rule....
1 pts Question 10 Determine if each of the statements is True or False about the resonance hybrid of C032. 1) All three resonance structures of CO3 contribute equally to the resonance hybrid of CO2. [Select ] 2) The central atom, C, has as a formal charge of -2 in the resonance hybrid. Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select) Select 4) The resonance hybrid follows the octet rule....
Draw the Lewis structure for NO2- including any valid resonance structures. Which of the following statements...
Draw the Lewis structure for NO2- including any valid resonance structures. Which of the following statements is TRUE? Draw the Lewis structure for NO2- including any valid resonance structures. Which of the following statements is TRUE? The nitrite ion contains two N=O double bonds. The nitrite ion contains one N
Most questions answered within 3 hours.
-
An infinite sheet of charge that has a surface charge density of
39 nC/m2 lies in...
asked 16 minutes ago -
After watching the lectures I am still having a hard time
understanding these equations.
1. The...
asked 1 hour ago -
Testing:
H0:μ=56.9
H1:μ<56.9
Your sample consists of 23 subjects, with a mean of 56.3 and
standard...
asked 1 hour ago -
straight wire, labeled as Wire A, lies horizontally on a
tabletop and is oriented to run...
asked 1 hour ago -
For a Chi-Squared Goodness of Fit Test about a uniform
distribution, complete the table.
Round to...
asked 1 hour ago -
Milk of magnesia is only slightly soluble in water. Kc for the
reaction Mg(OHs)(s)>Mg2+(a) + 2OH-1(aq)...
asked 1 hour ago -
Excel's HYPGEOM.DIST function can be used to compute _____.
a. both hypergeometric probabilities and cumulative
hypergeometric...
asked 2 hours ago -
The rocket used for the human lunar missions was the Saturn V
rocket. At liftoff the...
asked 1 hour ago -
1. The East Street Bagel Shop has a weekly demand for 3,500
bagels, which they make...
asked 1 hour ago -
paraphrasing each paragraph
“Where two or more persons knowingly act together unlawfully,
the act of each...
asked 2 hours ago -
The lateral line system in fish is contained which of the
following?
chemoreceptors
photoreceptors
mechanoreceptors
odor...
asked 2 hours ago -
Find the y-intercept of y =-5 x. The y-intercept of y equals
negative 5 x is______...
asked 2 hours ago
 Which of the following statements are true concerning CO32? 2-2 All carbon-oxygen bonds are equal in bond length. There are two sigma bonds in CO32 The bond angles about the central carbon atom are about 109°. There are two resonance structures for CO32 The central carbon atom is sp2 hybridized. true
Which of the following statements are true concerning CO32? 2-2 All carbon-oxygen bonds are equal in bond length. There are two sigma bonds in CO32 The bond angles about the central carbon atom are about 109°. There are two resonance structures for CO32 The central carbon atom is sp2 hybridized. true
 Determine if each of the statements is True or False about the resonance hybrid of PO4-. 1) The central atom, P, has one lone pair electrons. (Select] 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43-. [Select] 5)...
Determine if each of the statements is True or False about the resonance hybrid of PO4-. 1) The central atom, P, has one lone pair electrons. (Select] 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43-. [Select] 5)...
 Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of NO3 1) There are three equivalent resonance structures of NO3. Select 1 2) All three NO (nitrogen oxygen) bonds are equivalent in the resonance hybrid. I Select 1 3) The resonance hybrid violates the octet rule. Select 1 4) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. Select 1 5) There are two single...
Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of NO3 1) There are three equivalent resonance structures of NO3. Select 1 2) All three NO (nitrogen oxygen) bonds are equivalent in the resonance hybrid. I Select 1 3) The resonance hybrid violates the octet rule. Select 1 4) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. Select 1 5) There are two single...
 Determine if each of the statements is True or False about the resonance hybrid of PO43- [Select] 1) The central atom, P, has one lone pair electrons. 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. [Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. [Select] 4) All four resonance structures of PO43 contribute equally to the resonance hybrid of PO3- [Select ]...
Determine if each of the statements is True or False about the resonance hybrid of PO43- [Select] 1) The central atom, P, has one lone pair electrons. 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. [Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. [Select] 4) All four resonance structures of PO43 contribute equally to the resonance hybrid of PO3- [Select ]...
 Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of PO4 1) The central atom, P, has one lone pair electrons. True 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. True 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. True 4) All four resonance structures of PO43- contribute equally to the resonance hybrid...
Question 10 1 pts Determine if each of the statements is True or False about the resonance hybrid of PO4 1) The central atom, P, has one lone pair electrons. True 2) There are three single bonds and one double bond around the P atom in the resonance hybrid. True 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. True 4) All four resonance structures of PO43- contribute equally to the resonance hybrid...
 Determine if each of the statements is True or False about the resonance hybrid of PO43- 1) The central atom, P, has one lone pair electrons. [Select) 2) There are three single bonds and one double bond around the Patom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43- [Select] 5) The...
Determine if each of the statements is True or False about the resonance hybrid of PO43- 1) The central atom, P, has one lone pair electrons. [Select) 2) There are three single bonds and one double bond around the Patom in the resonance hybrid. (Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select] 4) All four resonance structures of PO43- contribute equally to the resonance hybrid of PO43- [Select] 5) The...
 Consider the resonance structures of formate. 0 Select the true statements about the resonance structures. Each oxygen atom has a double bond 50% of the time. The actual structure of formate is an average of the two resonance forms. The actual structure of formate switches back and forth between the two resonance forms. Each carbon-oxygen bond is somewhere between a single and double bond.
Consider the resonance structures of formate. 0 Select the true statements about the resonance structures. Each oxygen atom has a double bond 50% of the time. The actual structure of formate is an average of the two resonance forms. The actual structure of formate switches back and forth between the two resonance forms. Each carbon-oxygen bond is somewhere between a single and double bond.
 1 pts Question 10 Determine if each of the statements is True or False about the resonance hybrid of C032. 1) All three resonance structures of CO3 contribute equally to the resonance hybrid of CO2. [Select ] 2) The central atom, C, has as a formal charge of -2 in the resonance hybrid. Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select) Select 4) The resonance hybrid follows the octet rule....
1 pts Question 10 Determine if each of the statements is True or False about the resonance hybrid of C032. 1) All three resonance structures of CO3 contribute equally to the resonance hybrid of CO2. [Select ] 2) The central atom, C, has as a formal charge of -2 in the resonance hybrid. Select] 3) The formal charges on all terminal oxygen atoms are the same in the resonance hybrid. (Select) Select 4) The resonance hybrid follows the octet rule....