Homework Answers

Add Answer to:
When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot...
When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot...
 When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot engine has thermal efficiency e= 0.87 What is the coefficient of performance when it is operated as a Carnot refrigerator?
When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot engine has thermal efficiency e= 0.87 What is the coefficient of performance when it is operated as a Carnot refrigerator?
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write...
 8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
ed to a Carnot refrigerator so that all of the work produced by the engine is...
 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
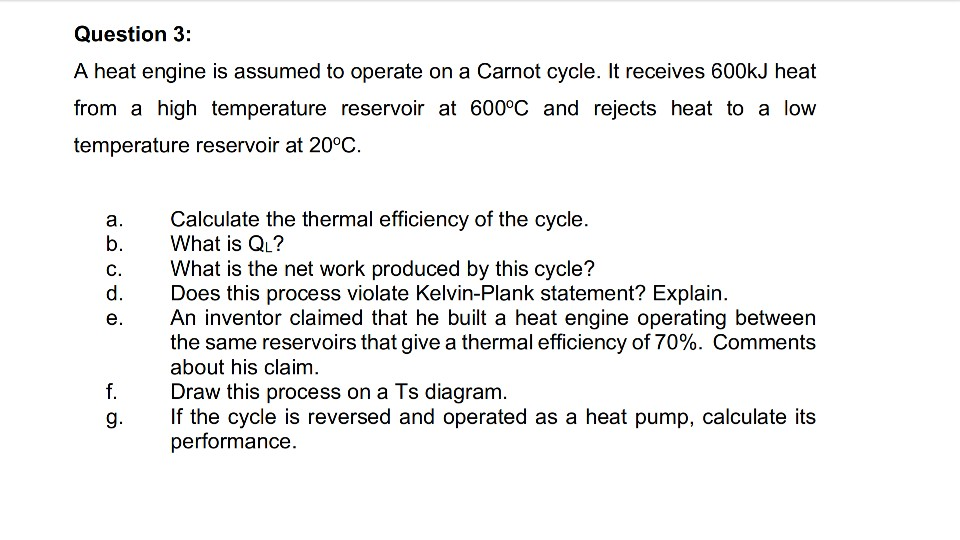 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308 K. (a) If the engine receives 5113 J of heat from the reservoir at T1 in each cycle, how many joules per cycle does it deliver to the reservoir at T2? (b) How much mechanical work is performed by the engine during each cycle? (c) What is the thermal efficiency of the engine?
A) A steam engine (assume a Carnot engine ) has an efficiency of 62.9%. If the...
A) A steam engine (assume a Carnot engine ) has an efficiency of 62.9%. If the waste heat has a temperature of 13.5◦C, what is the temperature of the boiler? Answer in units of ◦C. B) The interior of a refrigerator has a surface area of 2.4 m2. It is insulated by a 3 cm thick material that has a thermal conductivity of 0.0222 J/m · s ·◦ C. The ratio of the heat ex- tracted from the interior to...
A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in...
 A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in a P-V diagram, label the components, and then detail the processes that occur (I short phrase each is all that is necessary). Draw a diagram of the Carnot cycle in a H-S diagram, and label it to match (a) Note: His enthalpy and S is entropy. Water is brought to 0 °C in the refrigerator with the heat being discharged into a reservoir at...
A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in a P-V diagram, label the components, and then detail the processes that occur (I short phrase each is all that is necessary). Draw a diagram of the Carnot cycle in a H-S diagram, and label it to match (a) Note: His enthalpy and S is entropy. Water is brought to 0 °C in the refrigerator with the heat being discharged into a reservoir at...
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
Most questions answered within 3 hours.
-
What Volume (in mL) of KBr solution must be taken so it will
contain 2.83x10^-5 moles...
asked 12 minutes ago -
Dr Johnson invests $9,000 at the rate of 6% annual interest
compounded semi annually for 4...
asked 13 minutes ago -
The contribution format income statement for Huerra Company for
last year is given below:
Total
Unit...
asked 21 minutes ago -
How do I write a program in MIPs assembly that will take 2 words
from the...
asked 21 minutes ago -
two identical cars approach an intersection, one is traveling east
at 18n/s. the second is traveling...
asked 27 minutes ago -
When an X-ray beam with a wavelength of 133 pm is incident on
the surface to...
asked 36 minutes ago -
Compare and contrast the internal and external organization of
Congress and the President.
"2 paragraph"
asked 44 minutes ago -
Suppose that the average waiting time for a patient at a
physician's office is just over...
asked 1 hour ago -
C++
Can somebody help me with this
2 integer right triangles
There are right-angled triangles (i.e.,...
asked 1 hour ago -
A researcher at Lund University is interested in studying the
biological role of a so-called type...
asked 1 hour ago -
Consider where you currently work, where you have previously
worked, or a well-known company where you...
asked 1 hour ago -
For college physics discussion post. Be sure to have
examples.
Respond to the following:
Explain conservation...
asked 1 hour ago

 When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot engine has thermal efficiency e= 0.87 What is the coefficient of performance when it is operated as a Carnot refrigerator?
When a Carnot engine is operated in reversed direction it becomes a Carnot refrigerator. A Carnot engine has thermal efficiency e= 0.87 What is the coefficient of performance when it is operated as a Carnot refrigerator?
 8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
8: Answer to the following: a. Describe essential characteristics of the reversed Carnot refrigerator. b. Write down the formulas for the thermal efficiency of reversible heat engine and for the coefficient of performance (COP) of irreversible heat pump explicitly. Discuss the reason why the flow work of a heat pump must be minimized. d. Discuss which process is the best for a heat pump among the isentropic, polytropic, and isothermal processes in terms of the P-v property diagram.
 ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
ed to a Carnot refrigerator so that all of the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 0°C at the rate of 35 kJ-s . The source of energy for the Carnot engine is a heat reservoir 9.7. A Carnot engine is coupl at 250°C. If both devices discard heat to the surroundings at 25°C, how much heat does the engine absorb from its heat-source reservoir? If the...
 SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
SP3. A Carnot engine operating in reverse as a heat pump moves heat from a cold reservoir at 7°C to a warmer one at 22°C. a) What is the efficiency of a Carnot engine operating between these two temperatures? b) If the Carnot heat pump releases 250 J of heat into the higher-temperature reservoir e co in each cycle, how much work must be provided in each cycle? c) How much heat is removed from the 7°C reservoir in each...
 A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in a P-V diagram, label the components, and then detail the processes that occur (I short phrase each is all that is necessary). Draw a diagram of the Carnot cycle in a H-S diagram, and label it to match (a) Note: His enthalpy and S is entropy. Water is brought to 0 °C in the refrigerator with the heat being discharged into a reservoir at...
A refrigerator is constructed with a Carnot engine. Draw a diagram of the Carnot cycle in a P-V diagram, label the components, and then detail the processes that occur (I short phrase each is all that is necessary). Draw a diagram of the Carnot cycle in a H-S diagram, and label it to match (a) Note: His enthalpy and S is entropy. Water is brought to 0 °C in the refrigerator with the heat being discharged into a reservoir at...
 4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?
4. A Carnot engine works between two heat reservoirs at temperatures Ty 300 K & Te -77.0 a. What is its efficiency? b. If it absorbs c. How much heat does it release to the low- d. Wha 100 J of heat from the hot reservoir during each cycle, how much work does it do? t is the coefficient of performance of this engine when it works as a refrigerator between temperature reservoir during each cycle? these two reservoirs?