A heat engine that receives heat from a furnace at 1200 degrees C and rejects waste...
A heat engine that receives heat from a furnace at 1200 degrees C and rejects waste heat to a river at 20 degrees C has a thermal efficiency of 40 percent. Determine the second-law efficiency of this power plant. Show all work, including interpolation, and assumptions made. Please be neat.
Homework Answers
Add Answer to:
A heat engine that receives heat from a furnace at 1200 degrees
C and rejects waste...
A reversible heat engine receives heat of 2000 kJ from a furnace at temperature of 600...
A reversible heat engine receives heat of 2000 kJ from a furnace at temperature of 600 0C and rejects waste heat into the house. The portion of work produced by this heat engine utilized to drive a reversible heat pump to warmup the same house during the winter. The house is to be maintained at 21 0C at all times even though outside temperature drops to -15 0C. If the net-work output of the combined heat engine and heat pump...
A furnace is transferring heat to a heat engine from at a rate of 100 MW....
 A furnace is transferring heat to a heat engine from at a rate of 100 MW. Waste heat is rejected to a nearby river at a rate of 60 MW. Calculate the thermal efficiency for this heat engine. Ox-100MW 0= 60 MW Fig 1. Heat engine 24% a. 3496 ob. 36% OC. d. 4096 46%
A furnace is transferring heat to a heat engine from at a rate of 100 MW. Waste heat is rejected to a nearby river at a rate of 60 MW. Calculate the thermal efficiency for this heat engine. Ox-100MW 0= 60 MW Fig 1. Heat engine 24% a. 3496 ob. 36% OC. d. 4096 46%
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of...
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
An inventor claims to have perfected a heat engine that receives energy by means of heat...
An inventor claims to have perfected a heat engine that receives energy by means of heat transfer from a fuel at 300°C and rejects energy to the surroundings at 15°C while achieving a thermal efficiency of 60 percent. How would you evaluate the inventor's claims?
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
(43) A power plant receives heat from a 900 K reservoir and rejects heat to a...
 (43) A power plant receives heat from a 900 K reservoir and rejects heat to a sink at 300 K. The work produced is 300 MW from 550 MW of heat transferred. Water enters the boiler at 198 kg/s, 100°C, and 5 MPa; it is isobarically heated in the boiler. What are the theoretical and actual efficiencies of the heat engine? What is the outlet temperature of the water exiting the boiler? 3.
(43) A power plant receives heat from a 900 K reservoir and rejects heat to a sink at 300 K. The work produced is 300 MW from 550 MW of heat transferred. Water enters the boiler at 198 kg/s, 100°C, and 5 MPa; it is isobarically heated in the boiler. What are the theoretical and actual efficiencies of the heat engine? What is the outlet temperature of the water exiting the boiler? 3.
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
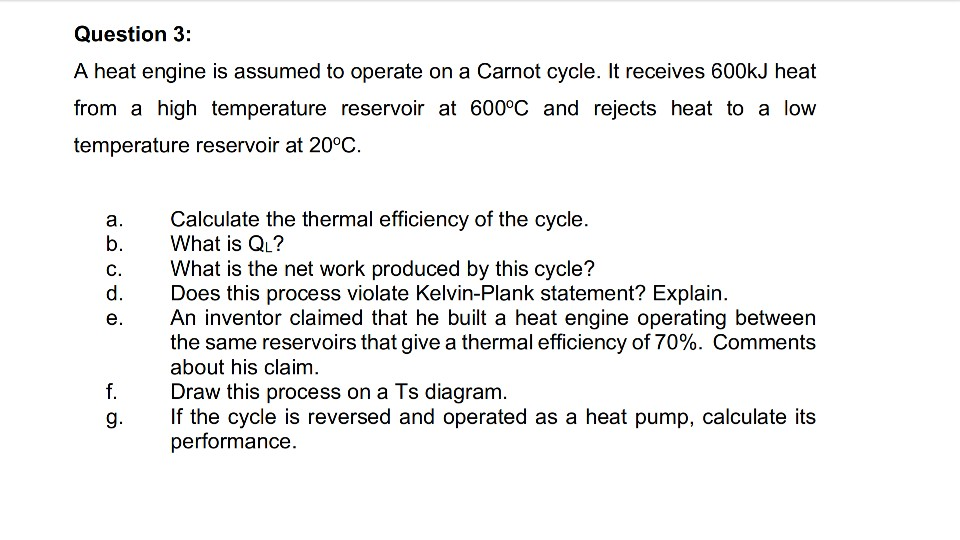 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
thermodynamics ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It...
 thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at...
 (12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
EXAM 3B 1. The Carnot heat engine receives 6500 kJ/h of heat from a boiler at...
 EXAM 3B 1. The Carnot heat engine receives 6500 kJ/h of heat from a boiler at 950 °C and rejects the waste heat to a well at 20°C. The produced power output of heat engine is fully used to operate the heat pump that draws the heat from the outdoor environment and transfers it to warm the house at the rate of 1100 kJ/min. If the house is maintained at 23°C, what is the lowest temperature of outdoor environment? (6...
EXAM 3B 1. The Carnot heat engine receives 6500 kJ/h of heat from a boiler at 950 °C and rejects the waste heat to a well at 20°C. The produced power output of heat engine is fully used to operate the heat pump that draws the heat from the outdoor environment and transfers it to warm the house at the rate of 1100 kJ/min. If the house is maintained at 23°C, what is the lowest temperature of outdoor environment? (6...
Most questions answered within 3 hours.
-
what's the danger in the fact that the market value of a stock
is based on...
asked 1 second ago -
Describe how do you feel about the post below and
why?
Listening to the podcast reaffirmed...
asked 8 minutes ago -
To start an avalanche on a mountain slope, an artillery shell is
fired with an initial...
asked 21 minutes ago -
The population of bacteria in a culture can be modeled by P left
parenthesis t right...
asked 25 minutes ago -
Which factors can prevent permanent fixation of an allele (i.e.
maintain genetic diversity)? Hint: You're going...
asked 28 minutes ago -
Compare a two-year bond with two successive one-year bonds in a
situation in which an investor...
asked 49 minutes ago -
Chapter 6
Search the internet and find a newspaper example of a price
ceiling, price floor...
asked 44 minutes ago -
Sarah Bates, calendar year taxpayer, started a new business on
October 8th. A number of start-up...
asked 45 minutes ago -
You and your friends are playing in the swimming pool with a
40-cm-diameter beach ball. How...
asked 50 minutes ago -
Patterson Development sometimes sells property on an installment
basis. In those cases, Patterson reports income in...
asked 1 hour ago -
please help with these two example, i want to double check my
work. thanks
1.
sum:=0...
asked 59 minutes ago -
in the formation of 1.0 mole of the following crystalline solids
from the gaseous ions most...
asked 1 hour ago

 A furnace is transferring heat to a heat engine from at a rate of 100 MW. Waste heat is rejected to a nearby river at a rate of 60 MW. Calculate the thermal efficiency for this heat engine. Ox-100MW 0= 60 MW Fig 1. Heat engine 24% a. 3496 ob. 36% OC. d. 4096 46%
A furnace is transferring heat to a heat engine from at a rate of 100 MW. Waste heat is rejected to a nearby river at a rate of 60 MW. Calculate the thermal efficiency for this heat engine. Ox-100MW 0= 60 MW Fig 1. Heat engine 24% a. 3496 ob. 36% OC. d. 4096 46%
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
 (43) A power plant receives heat from a 900 K reservoir and rejects heat to a sink at 300 K. The work produced is 300 MW from 550 MW of heat transferred. Water enters the boiler at 198 kg/s, 100°C, and 5 MPa; it is isobarically heated in the boiler. What are the theoretical and actual efficiencies of the heat engine? What is the outlet temperature of the water exiting the boiler? 3.
(43) A power plant receives heat from a 900 K reservoir and rejects heat to a sink at 300 K. The work produced is 300 MW from 550 MW of heat transferred. Water enters the boiler at 198 kg/s, 100°C, and 5 MPa; it is isobarically heated in the boiler. What are the theoretical and actual efficiencies of the heat engine? What is the outlet temperature of the water exiting the boiler? 3.
 thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
thermodynamics
ne operating between the heat source and heat sink temperatures of 16000K and 4000K. It draws 100 A heat engi MW of energy from the heat source and rejects 60 MW of energy to the heat sink. (a) What is the work output of this heat engine if the thermal efficiency is 40% (b) What is the second law efficiency of this heat engine (c) Is this heat engine compliant with the Second Law of Thermodynamics and the increase...
 (12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
(12 pts) A heat pump operating on a cyclic process receives heat from a reservoir at 500°C and rejects the waste heat at a rate of 30 kW to the ambient air at 300 K. If the work output of the engine is 45 kw, determine if the cycle is possible, and if the cycle is reversible. Explain with calculations. Show calculations using both efficiency (Method 1) and Entropy generation (Method 2) analysis.
 EXAM 3B 1. The Carnot heat engine receives 6500 kJ/h of heat from a boiler at 950 °C and rejects the waste heat to a well at 20°C. The produced power output of heat engine is fully used to operate the heat pump that draws the heat from the outdoor environment and transfers it to warm the house at the rate of 1100 kJ/min. If the house is maintained at 23°C, what is the lowest temperature of outdoor environment? (6...
EXAM 3B 1. The Carnot heat engine receives 6500 kJ/h of heat from a boiler at 950 °C and rejects the waste heat to a well at 20°C. The produced power output of heat engine is fully used to operate the heat pump that draws the heat from the outdoor environment and transfers it to warm the house at the rate of 1100 kJ/min. If the house is maintained at 23°C, what is the lowest temperature of outdoor environment? (6...