An inventor claims to have perfected a heat engine that receives energy by means of heat...
An
inventor claims to have perfected a heat engine that receives
energy by means of heat transfer from a fuel at 300°C and rejects
energy to the surroundings at 15°C while achieving a thermal
efficiency of 60 percent. How would you evaluate the inventor's
claims?
Homework Answers

Add Answer to:
An inventor claims to have perfected a heat engine that receives energy by means of heat...
The answer is NO but why? An inventor claims to have developed a heat engine that...
 The answer is NO but
why?
An inventor claims to have developed a heat engine that receives 700 kJ of heat from a source at 500 K and produces 300 kJ of net work while rejecting the waste heat to a sink at 290 K. Is this a reasonable claim? Yes or No Yes No
The answer is NO but
why?
An inventor claims to have developed a heat engine that receives 700 kJ of heat from a source at 500 K and produces 300 kJ of net work while rejecting the waste heat to a sink at 290 K. Is this a reasonable claim? Yes or No Yes No
An inventor claims he has developed a heat engine that receives energy from a source and...
 An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. A. Inventors claim violates both Kelvin-Planck and First law of thermodynamics B. Inventors claim violates first law of thermodynamics C. The inventors claim violates kelvin-Planck statement D. Inventors claim violates Clausius statement
An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. A. Inventors claim violates both Kelvin-Planck and First law of thermodynamics B. Inventors claim violates first law of thermodynamics C. The inventors claim violates kelvin-Planck statement D. Inventors claim violates Clausius statement
An inventor claims he has developed a heat engine that receives energy from a source and...
 An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. O A. The inventors claim violates kelvin-Planck statement B. Inventors claim violates Clausius statement C. Inventors claim violates both Kelvin-Planck and First law of thermodynamics D. Inventors claim violates first law of thermodynamics
An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. O A. The inventors claim violates kelvin-Planck statement B. Inventors claim violates Clausius statement C. Inventors claim violates both Kelvin-Planck and First law of thermodynamics D. Inventors claim violates first law of thermodynamics
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
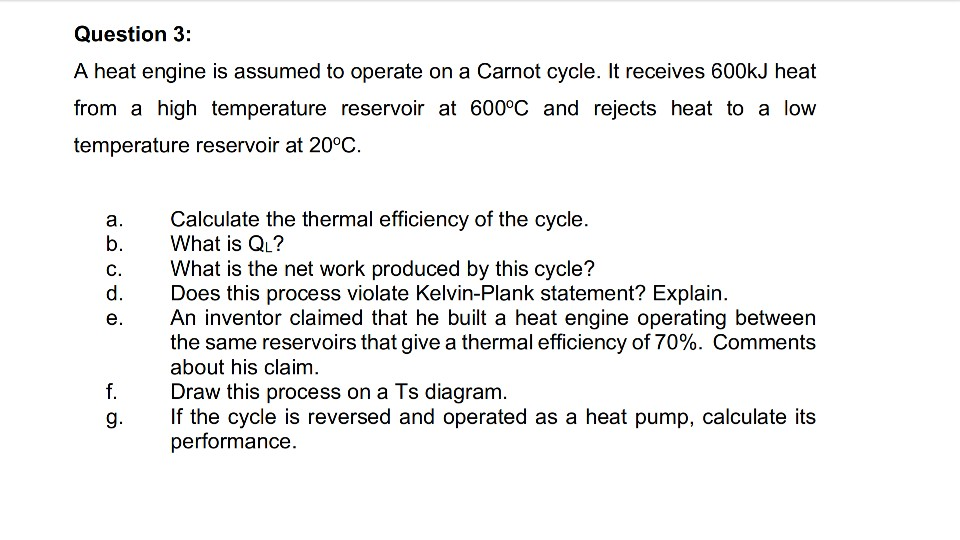 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
A heat engine that receives heat from a furnace at 1200 degrees C and rejects waste...
A heat engine that receives heat from a furnace at 1200 degrees C and rejects waste heat to a river at 20 degrees C has a thermal efficiency of 40 percent. Determine the second-law efficiency of this power plant. Show all work, including interpolation, and assumptions made. Please be neat.
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of...
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
An inventor claims to have devised a closed cyclic engine which exchanges heat with cold and...
An inventor claims to have devised a closed cyclic engine which exchanges heat with cold and hot reservoirs at 25 and 350 ◦C, respectively, and produces 0.45 kJ of work for every kJ of heat extracted from the hot reservoir. Is this claim believable?
As shown in the figure, a reversible power cycle receives energy QH by heat transfer from...
 As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
Most questions answered within 3 hours.
-
Here is a pair of aromatic compounds that each have a complicated
set of aromatic peaks....
asked 23 seconds from now -
As with many public goods, determining the appropriate level of
government support for the production of...
asked 2 minutes ago -
Write a balanced chemical equation for the oxidation reaction
between Caro’s acid (H2SO5) and CN– ion...
asked 5 minutes ago -
Calculate the number of valence electrons for
[Mo(C6H6)(CO)3]
asked 16 minutes ago -
Which of the following are true about the strength of the
association between two variables?
1-...
asked 12 minutes ago -
You are a monopolist and know that your consumers have a linear
demand curve. At what...
asked 11 minutes ago -
Raising a heavy load requires work. Raising it in half the time
requires:
asked 18 minutes ago -
MGT-201: Marketing Management
Before deciding on the structure of the channel marketers must
take several factors...
asked 17 minutes ago -
1. ACLs allow a router to control traffic within a network. The
concept is very similar...
asked 19 minutes ago -
35. When the electron in a hydrogen atom falls from its first
excited energy level to...
asked 21 minutes ago -
A man is dragging a box of mass "m" by using a rope which rests
on...
asked 37 minutes ago -
Liquid octane
(CH3(CH2)6CH3) will
react with gaseous oxygen (O2) to produce gaseous carbon
dioxide (CO2) and...
asked 38 minutes ago
 The answer is NO but
why?
An inventor claims to have developed a heat engine that receives 700 kJ of heat from a source at 500 K and produces 300 kJ of net work while rejecting the waste heat to a sink at 290 K. Is this a reasonable claim? Yes or No Yes No
The answer is NO but
why?
An inventor claims to have developed a heat engine that receives 700 kJ of heat from a source at 500 K and produces 300 kJ of net work while rejecting the waste heat to a sink at 290 K. Is this a reasonable claim? Yes or No Yes No
 An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. A. Inventors claim violates both Kelvin-Planck and First law of thermodynamics B. Inventors claim violates first law of thermodynamics C. The inventors claim violates kelvin-Planck statement D. Inventors claim violates Clausius statement
An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. A. Inventors claim violates both Kelvin-Planck and First law of thermodynamics B. Inventors claim violates first law of thermodynamics C. The inventors claim violates kelvin-Planck statement D. Inventors claim violates Clausius statement
 An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. O A. The inventors claim violates kelvin-Planck statement B. Inventors claim violates Clausius statement C. Inventors claim violates both Kelvin-Planck and First law of thermodynamics D. Inventors claim violates first law of thermodynamics
An inventor claims he has developed a heat engine that receives energy from a source and can completely transform this energy to work without any heat rejection. O A. The inventors claim violates kelvin-Planck statement B. Inventors claim violates Clausius statement C. Inventors claim violates both Kelvin-Planck and First law of thermodynamics D. Inventors claim violates first law of thermodynamics
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
 21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
21 A Carnot heat engine receives heat from a reservoir at 700°C at a rate of 6 MW and rejects the waste heat to the ambient at 300 K, as shown in the figure. The entire net power output of the heat engine is used to drive a Carnot refrigerator that removes heat from the cold medium at -10°C and transfers it to the same ambient at 300 K. Determine:35235333333 a) The thermal efficiency of the heat engine. b) The...
 As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
As shown in the figure, a reversible power cycle receives energy
QH by heat transfer from a hot reservoir at TH and rejects energy
QC by heat transfer to a cold reservoir at TC.
a) If TH = 1600 K, TC = 400 K, what is the thermal
efficiency?
b) If TH = 500oC, TC = 20oC, and Wcycle = 1000 kJ, what are QH and
QC, each in kJ?
c) If ? = 60% and TC = 40oF, what...
 IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...
IUDI ASSO 5.1 What is the highest cycle etlicy ssible for a heal nie pevati in 15 C 5.2 The reversible heat engines operate in series between a source at 527°C and a sink at 17°C if the engines have cqual eliciencies and the rest rejects 400J to the second, calculate: the Imperature at which lica is supplied to the second cagine: (ii) the heat taken from the source, (iii) the work done by cach engine Assume that each engine...