Homework Answers
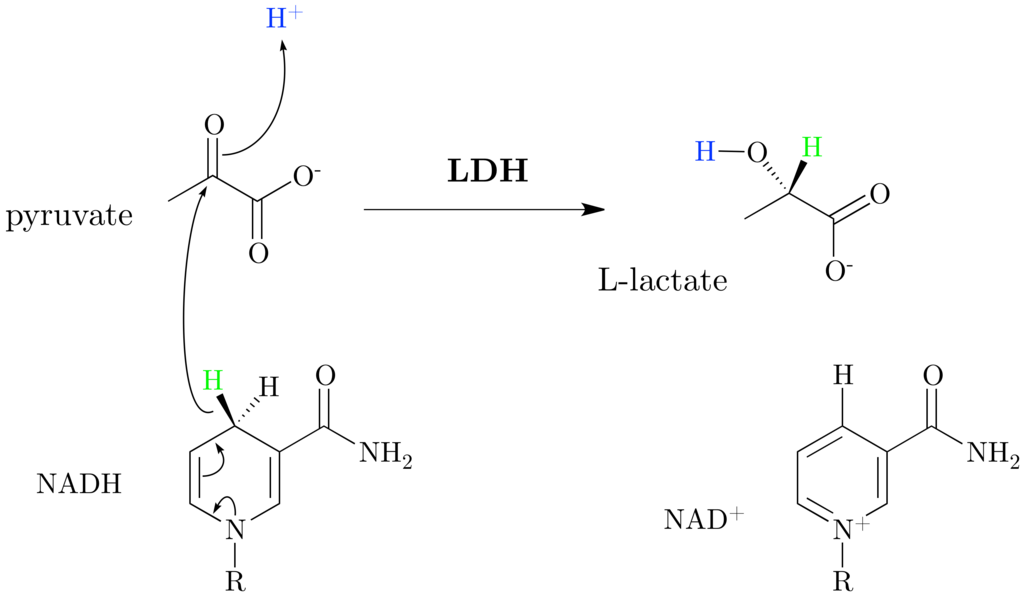
1)L-lactate dehydrogenase (L-LDH) catalyzes the
interconversion of pyruvate and NADH+ to L-lactate and NAD+.
H-lactate dehydrogenase (H-LDH) catalyzes the
interconversion of D-lactate and ferricytochrome c to pyruvate and
ferrocytochrome c.
Lactate Dehydrogenase (LDH) is an important enzyme in humans. It
occurs in different regions of the body, each region having a
unique conformation of different subunits. LDH is a key enzyme in
anaerobic respiration. Anaerobic Respiration is the
conversion of pyruvate into lactate acid in the
absence oxygen. This pathway is important to glycolysis in two main
ways. The first is that if pyruvate were to build up glycoysis and
thus the generation of ATP would slow. The second is anaerobic
respiration allows for the regeneration of NAD+ from NADH. NAD+ is
required when glyceraldehyde-3-phosphate dehydrogenase oxidizes
glyceraldehyde-3-phosphate in glycolysis, which generates NADH.
Lactate dehydrogenase is responsible for the anaerobic conversion
of NADH to NAD+.



An enzyme balances two apparently conflicting requirements to function properly. In forming the so-called Michaelis complex, the bound substrate is positioned within the protein in close contact with key protein groups that facilitate catalysis. Additionally for bimolecular reactions, the two bound substrates are held tightly together and positioned correctly for chemical reaction. Generally, static pictures involving no motion of the reacting groups are used to yield working mechanistic pictures. On the other hand, a second requirement of enzymatic catalysis is effective substrate binding and, the reverse, product release. Substrate is captured from solution and shuttled in and out of the binding pocket of the active site in a timely manner, typically on the order of a millisecond. Binding is necessarily a dynamical process. Substantial motions within the protein complex are required, including the recruitment of key proteins groups into the active site and desolvation and closure of the binding pocket. This often involves the motion of an active site loop, wherein an open form can facilitate ligand binding and release, and a closed form prepares, controls, and protects the reacting species. The dynamics of ligand binding to proteins is little understood, but involve motions from femtoseconds to tens of milliseconds (and sometimes even longer), and the process for enzymes is such that the enzyme·substrate complex lives just long enough to permit effective catalysis and no longer. The goal of this work is to examine the dynamics of how the Michaelis complex is formed in lactate dehydrogenase (LDH). We focus on how the enzyme·ligand encounter complex is formed; the encounter-complex species is of special importance in understanding the binding process.
LDH catalyzes the direct transfer of a hydride ion from the pro-R face of the reduced nicotinamide group of NADH to the C2 carbon of pyruvate producing NAD+ and the alcohol lactate, accelerating the solution chemical reaction by some 14 orders of magnitude . Binding of substrate to LDH is ordered and follows the formation of the LDH/NADH binary complex. The substrate binding pocket lies deep within the protein, buried ∼10 Å from the protein's surface , although our recent molecular dynamic calculations suggest that the protein samples conformations wherein the binding pocket is substantially exposed to solvent . It supplies the catalytically crucial His, and the preformed pocket additionally solvates the substrate's charged carboxyl group by supplying Arg. The rate-limiting step in the turnover of LDH is not the chemical hydride transfer step but rather loop motion involving closure of the so-called mobile loop (surface residues 98–110), occurring in a time of ∼1–10 ms

2) Many biological assays have as their basis a link to the oxidative status of nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate (NADP). Many dehydrogenase enzymes use these coenzymes to transfer hydrogen groups between molecules. Because the reduced forms of these molecules differ from the oxidized forms in their ability to absorb light, it is possible to quantitate reactions based on light absorbance at 340 nm or by the fluorescent emission of light at 445 nm. Here we describe the use of the Synerg 2 Multi-Detection Microplate Reader to quantitate NADH using either fluorescence or absorbance modes. Introduction Nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) are soluble dinucleotides that can be reversibly reduced by the addition of 2 hydrogen ions. While both molecules act as coenzymes in reversible reactions, they are involved in different types of reactions. NAD is generally used as an acceptor of reducing equivalents in catabolism, particularly glycolysis, the tricarboxylic acid cycle, and β-oxidation of fatty acids, while NADH, is reoxidized by complex I of the electron transport chain or by dehydrogenase enzymes during anaerobic metabolism (1). NADP is characteristically involved with reductive synthesis reactions, such as fatty acid and steroid synthesis (1). As demonstrated in Figure 1, NAD is a multiple ringed structure, which undergoes redox reactions within its nicotinamide ring (2). The closely related NADP molecule is phosphorylated on the 2’ position of the adenosine ribose ring. (2). In terms of quantitation, enzymatic dehydrogenase reactions involving NAD or NADP take advantage of the property of the reduced forms, NADH or NADPH, to absorb light at a wavelength of 340 nm while the oxidized forms do not. Likewise, the reduced forms are capable of fluorescent emission at 445 nm when excited at 340 nm, while the oxidized forms are not (4)
Lactate dehydrogenase is the most important clinically of several dehydrogenases occurring in human serum. Lactate dehydrogenase is cytoplasmic in its cellular location and in any one tissue is composed of one or two of five possible isoenzymes. While many of its clinical applications involve quantification of one or more specific serum isoenzymes, an estimate of total LD is required usually. Lactate dehydrogenase catalyzes the reversible reaction: L-lactate + NAD+ in equilibrium pyruvate + NADH. The bidirectional reaction is monitored spectrophotometrically by measuring either the increase in NADH at 340 nm produced in the lactate-to-pyruvate reaction (L----P) or by the decrease in NADH at 340 nm produced in the pyruvate-to-lactate (P----L) reaction. Kinetic assay systems for the measurement of the reaction system in both directions are comprehensively reviewed as well as the standardization efforts proposed to date.
Add Answer to:
8. Practice 6: Determination of LDH activity A. Pre-lab questions: 1) Which substrate should be used...
lab question 1. What is the basis of the different purification methods? 2. What are some...
lab question 1. What is the basis of the different purification methods? 2. What are some of the factors the might have interfered with your results? 3. How might you improve the process to increase the yield and purity? lab process E. coli BL21 (DE3) cells were transformed with the pET Topo-1521 vector containing a reading frame encoding the green fluorescent protein (GFP). Cells were cultured in M9ZB media at 37°C until the absorbance at 600 nm reached 0.7, at...
Create graphs for Figures 1-4 (circled on pages 111 & 114) based on the data given in Tables ...
 Create graphs for Figures 1-4 (circled on pages 111 & 114)
based on the data given in Tables 2 & 4.
Lab # 8 Cellular Respiration and Fermentation I. Goals and Objectives At the completion of this laboratory exercise, students will be able to: A Differentiate between the intermediates and products of fermentation versus acrobic cellular respiration in yeast. Relate rates of fermentation with sugar availability in yeast. Utilize a reduction-oxidation dye to determine the effect of varying yeast concentration...
Create graphs for Figures 1-4 (circled on pages 111 & 114)
based on the data given in Tables 2 & 4.
Lab # 8 Cellular Respiration and Fermentation I. Goals and Objectives At the completion of this laboratory exercise, students will be able to: A Differentiate between the intermediates and products of fermentation versus acrobic cellular respiration in yeast. Relate rates of fermentation with sugar availability in yeast. Utilize a reduction-oxidation dye to determine the effect of varying yeast concentration...
Help on prelab NOTE! There are FOUR (4) pages in this pre-lab! PRE-LAB WORKSHEET FOR ENZYME...
 Help on prelab
NOTE! There are FOUR (4) pages in this pre-lab! PRE-LAB WORKSHEET FOR ENZYME LAB To be completed prior to the online prelab esercise 1 ) Suppose that the diagram below represents what occurs during a chemical reaction e) Which letter points tq the prodact? print e ore , d 3) (c) Which letter points to the enzyme? (d) Which letter points to the enzyme's active site? (e) Which letter points to the enzyme's substrate? Note: Some letters...
Help on prelab
NOTE! There are FOUR (4) pages in this pre-lab! PRE-LAB WORKSHEET FOR ENZYME LAB To be completed prior to the online prelab esercise 1 ) Suppose that the diagram below represents what occurs during a chemical reaction e) Which letter points tq the prodact? print e ore , d 3) (c) Which letter points to the enzyme? (d) Which letter points to the enzyme's active site? (e) Which letter points to the enzyme's substrate? Note: Some letters...
help please? this was the only other information given REPORT SHEET Determination of the Solubility-Product Constant...
 help please?
this was the only other information given
REPORT SHEET Determination of the Solubility-Product Constant for a Sparingly Soluble Salt EXPERIMENT 8 A. Preparation of a Calibration Curve Initial (Cro121 0.0024 M Absorbance 5 mL Volume of 0.0024 M K Cro Total volume 1. I mL 100 mL 2. 100ML 3. 10 mL 100ml 4. 15 mL 100 ML Molar extinction coefficient for [CrO2) [Cro,2) 2.4x100M 12x1044 2.4810M 3.6810M 0.04) 2037.37 0.85 1.13 2. 3. Average molar extinction coefficient...
help please?
this was the only other information given
REPORT SHEET Determination of the Solubility-Product Constant for a Sparingly Soluble Salt EXPERIMENT 8 A. Preparation of a Calibration Curve Initial (Cro121 0.0024 M Absorbance 5 mL Volume of 0.0024 M K Cro Total volume 1. I mL 100 mL 2. 100ML 3. 10 mL 100ml 4. 15 mL 100 ML Molar extinction coefficient for [CrO2) [Cro,2) 2.4x100M 12x1044 2.4810M 3.6810M 0.04) 2037.37 0.85 1.13 2. 3. Average molar extinction coefficient...
Based on the document below, 1. Describe the hypothesis Chaudhuri et al ids attempting to evaluate;...
 Based on the document below,
1. Describe the hypothesis Chaudhuri et al ids attempting to
evaluate; in other words, what is the goal of this paper? Why is he
writing it?
2. Does the data presented in the paper support the hypothesis
stated in the introduction? Explain.
3.According to Chaudhuri, what is the potential role of thew
alkaline phosphatase in the cleanup of industrial waste.
CHAUDHURI et al: KINETIC BEHAVIOUR OF CALF INTESTINAL ALP WITH PNPP 8.5, 9, 9.5, 10,...
Based on the document below,
1. Describe the hypothesis Chaudhuri et al ids attempting to
evaluate; in other words, what is the goal of this paper? Why is he
writing it?
2. Does the data presented in the paper support the hypothesis
stated in the introduction? Explain.
3.According to Chaudhuri, what is the potential role of thew
alkaline phosphatase in the cleanup of industrial waste.
CHAUDHURI et al: KINETIC BEHAVIOUR OF CALF INTESTINAL ALP WITH PNPP 8.5, 9, 9.5, 10,...
1. Describe the functions of the following reagents in extraction of DNA from corn meal: proteina...
 1. Describe the functions of the following reagents in extraction of DNA from corn meal: proteinase K; guanidine HCI; SDS 2. Why is the ratio of the OD at 260 and 280 nm used to estimate DNA purity? 3. In one paragraph, summarize basic principles of PCR technique in your own words. List all the reagents you will need to perform a PCR experiment. Does this method tell you what genetic modifications were made? If yes, describe how you can...
1. Describe the functions of the following reagents in extraction of DNA from corn meal: proteinase K; guanidine HCI; SDS 2. Why is the ratio of the OD at 260 and 280 nm used to estimate DNA purity? 3. In one paragraph, summarize basic principles of PCR technique in your own words. List all the reagents you will need to perform a PCR experiment. Does this method tell you what genetic modifications were made? If yes, describe how you can...
Most questions answered within 3 hours.
-
3. Gains from trade
Consider two neighbouring island countries called Euphoria and
Contente. They each have...
asked 32 minutes ago -
A business executive has the option to invest money in two
plans: Plan A guarantees that...
asked 2 hours ago -
Hello, can someone please help me answer this question?
How much heat is absorbed by a...
asked 2 hours ago -
. A marketing researcher conducted a survey of 25 shoppers
randomly selected at the local mall...
asked 3 hours ago -
Create an comprehensive response to the
following:
Antimicrobial agents work on a multitude of microbes (bacteria,...
asked 3 hours ago -
6.13 LAB: Step counter. Section 6.3.
A pedometer treats walking 2,000 steps as walking 1 mile....
asked 3 hours ago -
(14.2) A block of mass m = 10 kg riding on a frictionless
horizontal plane is...
asked 3 hours ago -
Use any search engine to search for articles about Starbucks
partnership with Tata Companies in India...
asked 3 hours ago -
Let’s say that for some reason Bank Excess Reserves suddenly
increase sharply. What effect would this...
asked 3 hours ago -
Given:
Curent Assets: $600,000
Total Assets: $2,600,000
Current Liabilities: $500,000
Total Liabilities: $1,700,000
What is the...
asked 3 hours ago -
1. What is a “Bankster”? What is insider trading? Why is it
illegal?
2. What is...
asked 3 hours ago -
A transverse wave on a cord is given by
D(x,t)=0.18sin(2.7x−61.0t), where Dand x are in m...
asked 3 hours ago


 Create graphs for Figures 1-4 (circled on pages 111 & 114)
based on the data given in Tables 2 & 4.
Lab # 8 Cellular Respiration and Fermentation I. Goals and Objectives At the completion of this laboratory exercise, students will be able to: A Differentiate between the intermediates and products of fermentation versus acrobic cellular respiration in yeast. Relate rates of fermentation with sugar availability in yeast. Utilize a reduction-oxidation dye to determine the effect of varying yeast concentration...
Create graphs for Figures 1-4 (circled on pages 111 & 114)
based on the data given in Tables 2 & 4.
Lab # 8 Cellular Respiration and Fermentation I. Goals and Objectives At the completion of this laboratory exercise, students will be able to: A Differentiate between the intermediates and products of fermentation versus acrobic cellular respiration in yeast. Relate rates of fermentation with sugar availability in yeast. Utilize a reduction-oxidation dye to determine the effect of varying yeast concentration...
 Help on prelab
NOTE! There are FOUR (4) pages in this pre-lab! PRE-LAB WORKSHEET FOR ENZYME LAB To be completed prior to the online prelab esercise 1 ) Suppose that the diagram below represents what occurs during a chemical reaction e) Which letter points tq the prodact? print e ore , d 3) (c) Which letter points to the enzyme? (d) Which letter points to the enzyme's active site? (e) Which letter points to the enzyme's substrate? Note: Some letters...
Help on prelab
NOTE! There are FOUR (4) pages in this pre-lab! PRE-LAB WORKSHEET FOR ENZYME LAB To be completed prior to the online prelab esercise 1 ) Suppose that the diagram below represents what occurs during a chemical reaction e) Which letter points tq the prodact? print e ore , d 3) (c) Which letter points to the enzyme? (d) Which letter points to the enzyme's active site? (e) Which letter points to the enzyme's substrate? Note: Some letters...
 help please?
this was the only other information given
REPORT SHEET Determination of the Solubility-Product Constant for a Sparingly Soluble Salt EXPERIMENT 8 A. Preparation of a Calibration Curve Initial (Cro121 0.0024 M Absorbance 5 mL Volume of 0.0024 M K Cro Total volume 1. I mL 100 mL 2. 100ML 3. 10 mL 100ml 4. 15 mL 100 ML Molar extinction coefficient for [CrO2) [Cro,2) 2.4x100M 12x1044 2.4810M 3.6810M 0.04) 2037.37 0.85 1.13 2. 3. Average molar extinction coefficient...
help please?
this was the only other information given
REPORT SHEET Determination of the Solubility-Product Constant for a Sparingly Soluble Salt EXPERIMENT 8 A. Preparation of a Calibration Curve Initial (Cro121 0.0024 M Absorbance 5 mL Volume of 0.0024 M K Cro Total volume 1. I mL 100 mL 2. 100ML 3. 10 mL 100ml 4. 15 mL 100 ML Molar extinction coefficient for [CrO2) [Cro,2) 2.4x100M 12x1044 2.4810M 3.6810M 0.04) 2037.37 0.85 1.13 2. 3. Average molar extinction coefficient...
 Based on the document below,
1. Describe the hypothesis Chaudhuri et al ids attempting to
evaluate; in other words, what is the goal of this paper? Why is he
writing it?
2. Does the data presented in the paper support the hypothesis
stated in the introduction? Explain.
3.According to Chaudhuri, what is the potential role of thew
alkaline phosphatase in the cleanup of industrial waste.
CHAUDHURI et al: KINETIC BEHAVIOUR OF CALF INTESTINAL ALP WITH PNPP 8.5, 9, 9.5, 10,...
Based on the document below,
1. Describe the hypothesis Chaudhuri et al ids attempting to
evaluate; in other words, what is the goal of this paper? Why is he
writing it?
2. Does the data presented in the paper support the hypothesis
stated in the introduction? Explain.
3.According to Chaudhuri, what is the potential role of thew
alkaline phosphatase in the cleanup of industrial waste.
CHAUDHURI et al: KINETIC BEHAVIOUR OF CALF INTESTINAL ALP WITH PNPP 8.5, 9, 9.5, 10,...
 1. Describe the functions of the following reagents in extraction of DNA from corn meal: proteinase K; guanidine HCI; SDS 2. Why is the ratio of the OD at 260 and 280 nm used to estimate DNA purity? 3. In one paragraph, summarize basic principles of PCR technique in your own words. List all the reagents you will need to perform a PCR experiment. Does this method tell you what genetic modifications were made? If yes, describe how you can...
1. Describe the functions of the following reagents in extraction of DNA from corn meal: proteinase K; guanidine HCI; SDS 2. Why is the ratio of the OD at 260 and 280 nm used to estimate DNA purity? 3. In one paragraph, summarize basic principles of PCR technique in your own words. List all the reagents you will need to perform a PCR experiment. Does this method tell you what genetic modifications were made? If yes, describe how you can...