A heat engine has an efficiency of 83.557% and receives 215.163J of heat per cycle. How...
A heat engine has an efficiency of 83.557% and receives 215.163J of heat per cycle. How much work does it perform in each cycle?
Homework Answers

Add Answer to:
A heat engine has an efficiency of 83.557% and receives 215.163J
of heat per cycle. How...
a heat engine has an efficiency of 35% and receives 150J
a heat engine has an efficiency of 35% and receives 150J of heat per cycle. How much heat does it exhaust in each cycle?
3. An ideal Carnot engine has an input of 150 J of heat per cycle at...
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
Engine 1 has an efficiency of 0.19 and requires 6000 J of input heat to perform...
Engine 1 has an efficiency of 0.19 and requires 6000 J of input heat to perform a certain amount of work. Engine 2 has an efficiency of 0.23 and performs the same amount of work. How much input heat does the second engine require?
Engine 1 has an efficiency of 0.10 and requires 5500 J of input heat to perform...
Engine 1 has an efficiency of 0.10 and requires 5500 J of input heat to perform a certain amount of work. Engine 2 has an efficiency of 0.30 and performs the same amount of work. How much input heat does the second engine require?
? Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives...
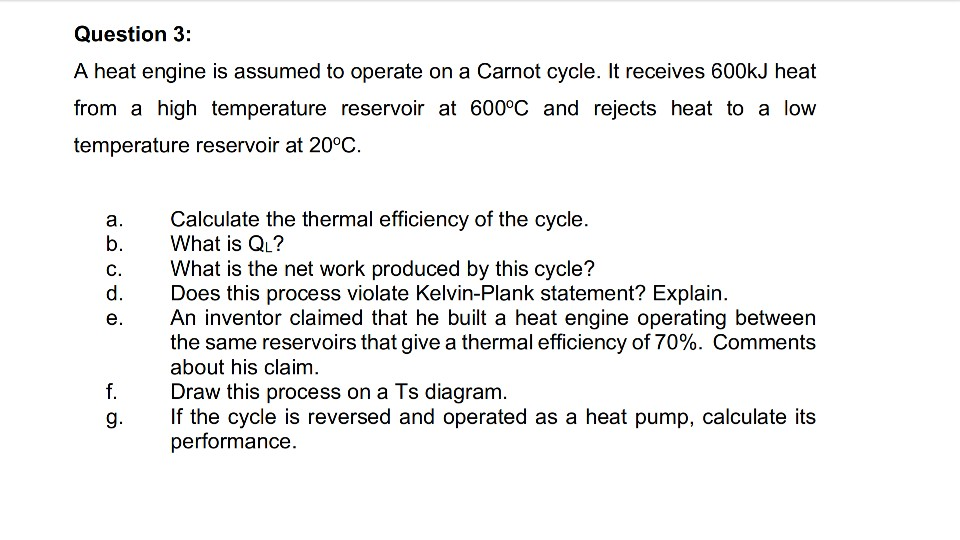 ?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
?
Question 3: A heat engine is assumed to operate on a Carnot cycle. It receives 600kJ heat from a high temperature reservoir at 600°C and rejects heat to a low temperature reservoir at 20°C. ooooo Calculate the thermal efficiency of the cycle. What is QL? What is the net work produced by this cycle? Does this process violate Kelvin-Plank statement? Explain. An inventor claimed that he built a heat engine operating between the same reservoirs that give a thermal...
1a. Engine (1) has an efficiency of 0.12 and requires 5490 J of input heat to perform...
1a. Engine (1) has an efficiency of 0.12 and requires 5490 J of input heat to perform a certain amount of work. Engine (2) has an efficiency of 0.28 and performs the same amount of work. How much input heat does the second engine require? b. An ideal heat engine takes in heat from a reservoir at 320 °C and has an efficiency of 28%. If the exhaust temperature does not vary and the efficiency is increased to 43%, what would...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308...
A Carnot engine is operated between two heat reservoirs at T1= 435 K and T2= 308 K. (a) If the engine receives 5113 J of heat from the reservoir at T1 in each cycle, how many joules per cycle does it deliver to the reservoir at T2? (b) How much mechanical work is performed by the engine during each cycle? (c) What is the thermal efficiency of the engine?
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300...
A Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it reject to the reservoir at 300 K? b) How much mechanical work is performed by the engine during each cycle? c) What is the thermal efficiency of the engine?
Most questions answered within 3 hours.
-
One tablet of Tums contains 350.0 mg of CaCo3. If 4 tablets of
Tums is added...
asked 1 minute ago -
Jason Allen is saving for an Australian vacation in three years.
He estimates that he will...
asked 22 minutes ago -
starting in the radial vein list in order all the vessels through
which an erythrocyte will...
asked 28 minutes ago -
Management at Work
As the training and development manager at the Litter*Bit*Better
Cat Litter Company, part...
asked 30 minutes ago -
What is the significance of the constraints in a physical model?
What part do distinct constraints...
asked 1 hour ago -
a student was diagnosed with myopia (nearshightedness). He can
comfortably read a newspaper when it is...
asked 1 hour ago -
Consider a 3m x 3m window in a house. The thermal conductivity
is reported to be...
asked 2 hours ago -
Why has California been the favorite destination of large number
of secondary migrants?
asked 2 hours ago -
Do not neglect the old for the new. The existing business must
not lose priority simply...
asked 5 hours ago -
Kylie is a single mom with two dependent children,
Tanner, age 7 and Olivia, age 11....
asked 6 hours ago -
Phosphorous + bromine = phosphorous tribromide. If 35.0 g of
bromine are reacted and 27.9 grams...
asked 8 hours ago -
Derive the long wavelength limit of the Planck energy density
distribution
asked 8 hours ago
 3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
3. An ideal Carnot engine has an input of 150 J of heat per cycle at its high-temperature reservoir, which is maintained at 135 °C. The engine has a thermal efficiency of 22.0%. a. How much work does this engine do per cycle? b. How much heat does this engine output to its low-temperature reservoir per cycle? c. What is the temperature of the low-temperature reservoir? d. How many cycles would this engine have to go through to lift a...
 Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
Solve the following problem in Thermodynamics: Carnot Cycle A heat engine receives heat from a source at 2000 K at a rate of 500 kW, and rejects the waste heat to a medium at 300 K. The net output from the engine is 300 kW. Determine the maximum energy that can be driven out of the engine theoretically using Carnot Cycle. Compare the observed work-efficiency with the expected efficiency of the heat engine? How much energy is lost due to...
 A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...
A certain heat engine operating on a Carnot cycle absorbs 370 J of heat per cycle at its hot reservoir at 145 degree C and has a thermal efficiency of 24.0% By how much does the engine change the entropy of the world each cycle? Express your answer to two significant figures and include the appropriate units. What mass of water could this engine pump per cycle from a well 25.0 m deep? Express your answer to two significant figures...